Catalyst, preparation method thereof and preparation method of amide compound
A technology of amide compounds and catalysts, applied in the field of catalyst preparation, which can solve the problems of restricting large-scale applications, large catalyst usage, and narrow substrate application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
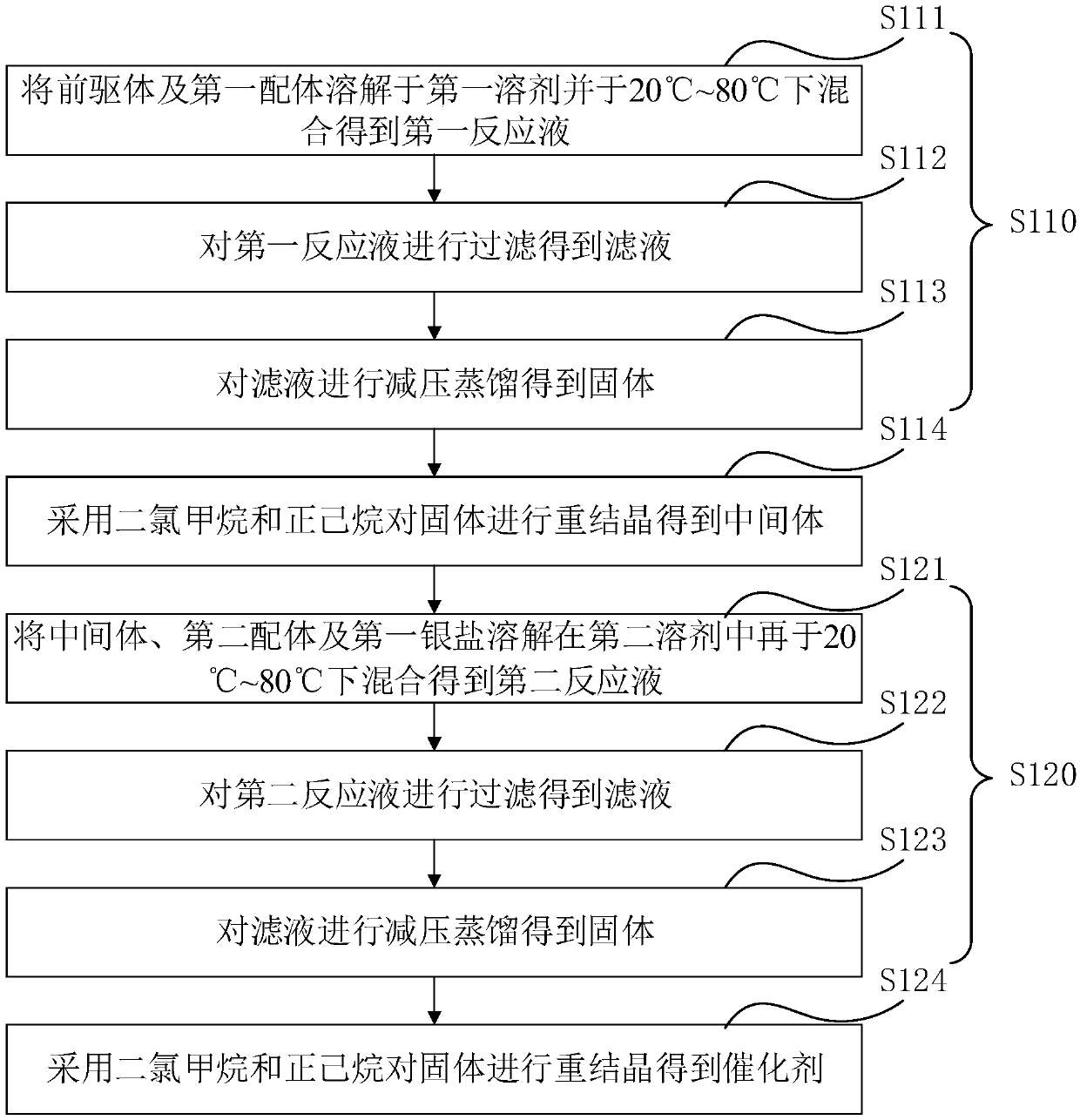
[0059] see figure 1 , the preparation method of the catalyst of one embodiment, comprises the following steps:
[0060] S110, reacting the precursor and the first ligand in the first solvent at 20° C. to 80° C. to obtain an intermediate.
[0061] In one embodiment, the step of mixing the precursor and the first ligand in the first solvent at 20°C to 80°C to obtain the intermediate specifically includes:
[0062] S111. Dissolving the precursor and the first ligand in the first solvent and mixing them at 20° C. to 80° C. to obtain a first reaction solution.
[0063] In one embodiment, the precursor is selected from at least one of (1,5-cyclooctadiene)platinum chloride and (1,5-cyclooctadiene)platinum bromide.
[0064] In one of the embodiments, the first ligand is selected from 1,1'-bis(diphenylphosphino)ferrocene, 1,1'-bis[(5-methyl-2-furyl)phosphino] Ferrocene, (-)-1,1-bis((2S,4S)-2,4-diethylphosphino)ferrocene, (-)-1,1-bis((2S,5S)-2 ,4-Dimethylphosphino)ferrocene, (1R)-1[...
Embodiment 1
[0121] The preparation method of the catalyst of the present embodiment is as follows:
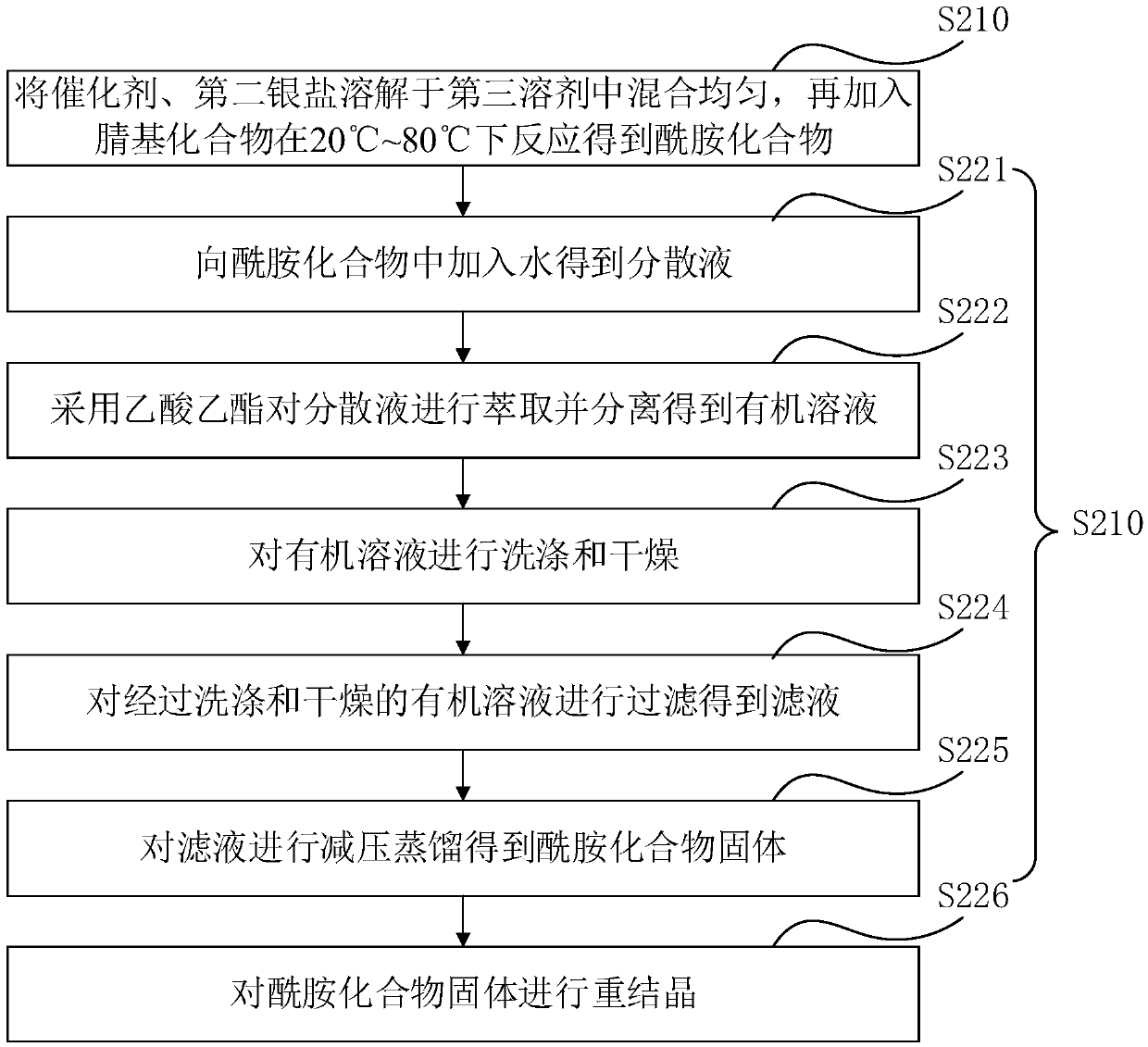
[0122] Dissolve (1,5-cyclooctadiene)platinum chloride and 1,1'-bis(diphenylphosphino)ferrocene in dichloromethane at a molar ratio of 1:1, and then proceed at 25°C Stirring for 12 hours to obtain the first reaction solution, filtering the first reaction solution to obtain a filtrate, and distilling the filtrate under reduced pressure to obtain a solid, dissolving the solid in dichloromethane, then adding n-hexane to it for recrystallization to obtain an intermediate, The volume ratio of dichloromethane to n-hexane is 1:1; the intermediate obtained above, dimethyl hydroxyphosphine and silver tetrafluoroborate are dissolved in dichloromethane, and then stirred at 25°C for 12h to obtain the second reaction liquid, wherein the molar ratio of the intermediate to dimethylhydroxyphosphorus is 1:1.05, and the molar ratio of the intermediate to silver tetrafluoroborate is 1:1; the second reaction l...
Embodiment 2
[0132] The preparation method of the catalyst of the present embodiment is as follows:
[0133] Dissolve (1,5-cyclooctadiene)platinum chloride and 1,1'-bis[(5-methyl-2-furyl)phosphino]ferrocene in dichloro In methane, stirred at 40°C for 4h to obtain the first reaction solution, filtered the first reaction solution to obtain a filtrate, and distilled the filtrate under reduced pressure to obtain a solid, dissolved the solid in dichloromethane, and then added N-hexane is recrystallized to obtain an intermediate, and the volume ratio of dichloromethane to n-hexane is 1:1; the intermediate obtained above, dimethyl hydroxyphosphine and silver trifluoromethanesulfonate are dissolved in dichloromethane, and then Stir at 40°C for 4h to obtain a second reaction solution, wherein the molar ratio of the intermediate to dimethylhydroxyphosphorus is 1:1.05, and the molar ratio of the intermediate to silver tetrafluoroborate is 1:1; the second reaction Liquid is filtered to obtain filtrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com