Method for producing 3-methyl-4-aminobenzoic acid through catalytic hydrogenation method
A technology of aminobenzoic acid and nitrobenzoic acid, which is applied in chemical instruments and methods, preparation of organic compounds, physical/chemical process catalysts, etc., can solve problems such as iron mud environmental pollution, poor working conditions, and time to wait. Achieve the effect of reducing environmental pressure, low cost and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Taking 200 kg of 3-methyl-4-nitrobenzoic acid as an example, the catalyst used is a self-made catalyst. The total amount of catalyst used in the production process is 0.1-3% (0.2-6 kg) of the raw material, and the total amount of water is the raw material 8 times (1600 kg), before hydrogenation, 3-methyl-4-nitrobenzoic acid, water and catalyst are put into the tank in the following order.
[0018] 1. Put 200 kg of 3-methyl-4-nitrobenzoic acid and 1600 kg of water into the reaction kettle, and adjust the pH value of the reaction solution to 7-8 by adding sodium hydroxide, potassium hydroxide, sodium carbonate or potassium carbonate, etc. , gas displacement.
[0019] 2. Introduce hydrogen to start the hydrogenation reaction, control the reaction temperature to 80±5°C, and control the reaction pressure to 0.3-1.0Mpa.
[0020] 3. After the reaction is finished, filter the catalyst, and add acidifying agents such as hydrochloric acid and sulfuric acid to the filtrate to adj...
Embodiment 2
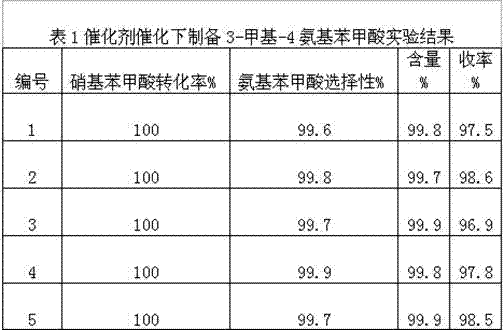
[0023] Taking 200 kg of 3-methyl-4-nitrobenzoic acid to produce 3-methyl-4-aminobenzoic acid as an example, the catalyst used in the production process is a noble metal catalyst (self-made), and the dosage is 3-methyl-4-nitrobenzoic acid 0.1-3% (0.2-6 kg) of benzoic acid, and the total amount of water is 8 times (1600 kg) of the raw material. In continuous production, 3-methyl-4-nitrobenzoic acid, water and catalyst are based on (1) was added in order. In order to investigate the stability of the process, five consecutive experiments were carried out, and the results are shown in Table 1 as follows:
[0024]
[0025] Reaction conditions: temperature 80±5°C, pressure 0.3-1.0Mpa, ph7-8.
[0026] As can be seen from the table, the conversion rate of 3-methyl-4-nitrobenzoic acid is 100%, the selectivity of 3-methyl-4-aminobenzoic acid is more than 99.5%, and the finished product content is more than 99.7%. Above 97%.
Embodiment 3
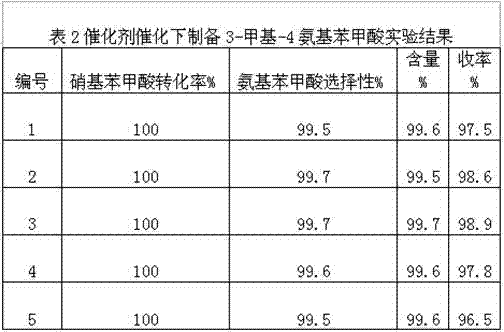
[0028] Taking 200 kilograms of 3-methyl-4-nitrobenzoic acid to produce 3-methyl-4-aminobenzoic acid as an example, the catalyst used in the production process is a noble metal catalyst, and the dosage is 3-methyl-4-nitrobenzoic acid 0.1-3% (0.2-6 kg), the total amount of water is 8 times (1600 kg) of the raw material, during continuous production, the reaction is carried out according to the production process of Example 1, the difference is that the catalyst is recycled. After the reaction in each kettle is completed, after the catalyst is filtered by pressure, the catalyst is blown back into the reaction kettle, and 0.1-0.5 kg of catalyst is added, and the catalyst is recycled. After the catalyst started to be used mechanically, the amount of new catalyst added was greatly reduced, only 5%-25% of the original, which greatly reduced the cost. After mass production experiments, this catalyst can be used continuously for more than ten times.
[0029] In order to reduce the amou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com