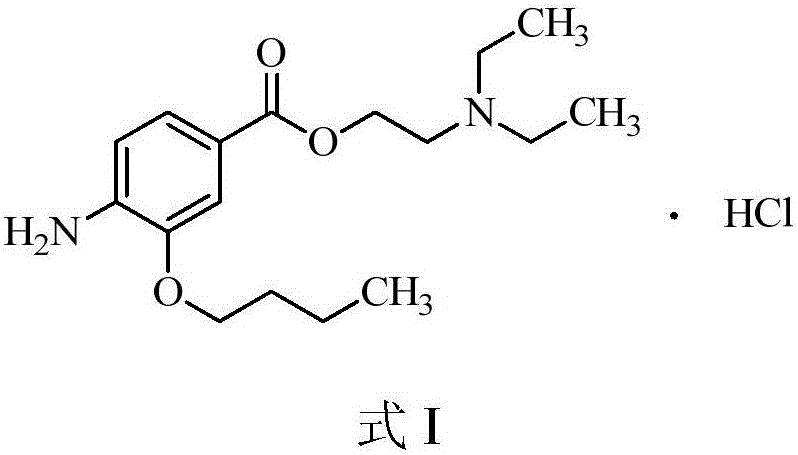
Preparation method of oxybuprocaine hydrochloride
A technology of obucaine and nitrobenzoic acid, applied in the field of compound synthesis, can solve the problems of high equipment requirements, unfavorable production, easy combustion and explosion, etc., and achieves the effect of easy cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, preparation oxybucaine hydrochloride
[0040] (1) Preparation of ethyl 3-butoxy-4-nitrobenzoate
[0041] Add 40g 3-hydroxyl-4-nitrobenzoic acid ethyl ester, 31g bromobutane, 31g anhydrous potassium carbonate and 400ml DMF in the there-necked flask, in the reaction system, 3-hydroxyl-4-nitrobenzoic acid ethyl ester, bromine The molar ratio of butane to potassium carbonate is 1:1.2:1.2; heat to 80°C, react for 12 hours, after the reaction is completed, remove the solvent by rotary evaporation, add 200ml of water to the residue, stir and crystallize for 12 hours, filter to obtain 52g of light yellow Solid 3-butoxy-4-nitro-benzoic acid ethyl ester.
[0042] (2) Preparation of 3-butoxy-4-nitro-benzoic acid
[0043] Add 52g of ethyl 3-butoxy-4-nitrobenzoate obtained in step (1) and 150ml of 15wt% sodium hydroxide solution into a three-necked flask, stir, heat at 60°C, react for 1h, cool down with ice water, and slowly add hydrochloric acid , adjusted the pH t...
Embodiment 2
[0050] Embodiment 2, preparation oxybucaine hydrochloride
[0051] (1) Preparation of ethyl 3-butoxy-4-nitrobenzoate
[0052] Add 400g 3-hydroxyl-4-nitrobenzoic acid ethyl ester, 310g bromobutane, 310g anhydrous potassium carbonate and 4000ml DMF in the there-necked flask, 3-hydroxyl-4-nitrobenzoic acid ethyl ester, bromobutane and carbonic acid The molar ratio of potassium is 1:1.2:1.2; heat to 80°C, react for 14 hours, after the reaction is completed, remove the solvent by rotary evaporation, add 2000ml of water to the residue, stir and crystallize for 12 hours, filter to obtain 531g of light yellow solid 3-butyl Oxy-4-nitro-benzoic acid ethyl ester.
[0053](2) Preparation of 3-butoxy-4-nitrobenzoic acid
[0054] Add 530g of 3-butoxy-4-nitro-benzoic acid ethyl ester obtained in step (1) and 1590ml of 15wt% sodium hydroxide solution into a three-necked flask, stir, heat to 60°C, react for 1h, cool down with ice water, slowly Hydrochloric acid was added to adjust the pH to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com