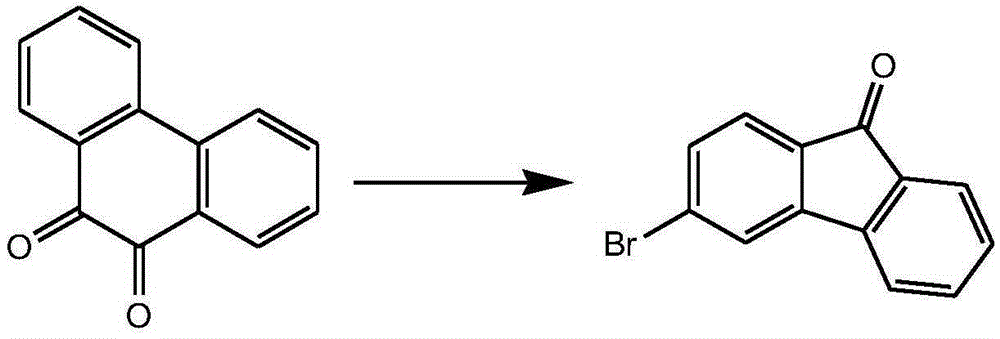
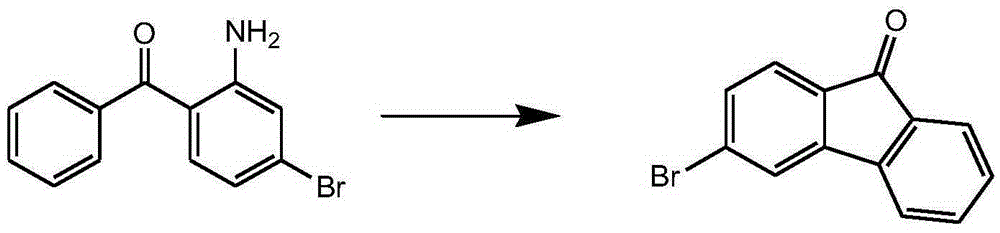
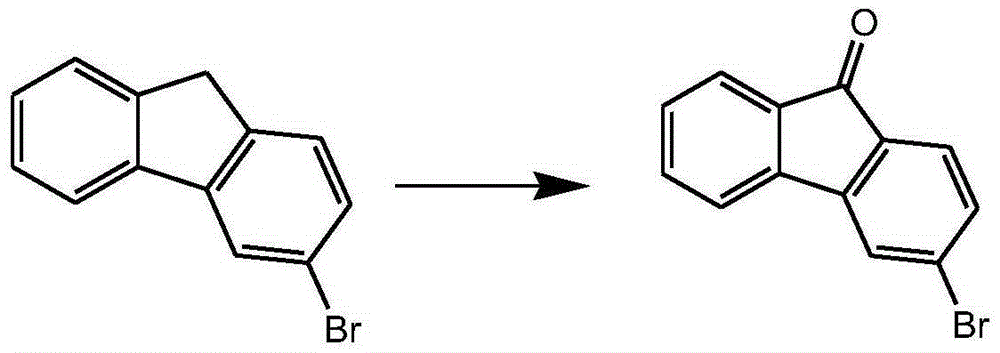
Preparation method of 3-halogenated fluorenone compound
A technology of halogenated fluorenones and compounds, which is applied in the field of preparation of 3-halogenated fluorenones, can solve the problems of 3-bromofluorenones being expensive and difficult to prepare, and achieve short cycle times, simple operations, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1) Preparation of Compound A
[0037]
[0038] Under nitrogen protection, 2-bromo-4-nitrobenzoic acid methyl ester (6.5g, 0.025mol), phenylboronic acid (3.2g, 0.0262mol) and 30% mass fraction of potassium carbonate aqueous solution ( Potassium carbonate: 8.637g, 0.0625mol; water: 20.16g), in solvent toluene (19.5g), catalyst palladium acetate (0.0056g, 7.5*10 -5 mol) and ligand triphenylphosphine (0.0393g, 1.5*10 -4 mol) in the presence of the mixture, the temperature was raised to 55-60° C., and the reaction was complete for 2 hours (TLC followed the reaction). After the reaction, the mixture was cooled to 0-40°C, the aqueous phase was separated by liquid separation, the organic phase was washed with water to pH = 7, dried over anhydrous sodium sulfate, passed through a silica gel column, and concentrated by rotary evaporation to obtain a brownish yellow solid, which was weighed with toluene Crystallized to obtain a pale yellow solid, dried in vacuo to obtain 5.7 ...
Embodiment 2
[0054] 1) Preparation of Compound A
[0055]
[0056] Under nitrogen protection, 2-bromo-4-nitrobenzoic acid methyl ester (6.5g, 0.025mol), phenylboronic acid (3.2g, 0.0262mol) and 30% mass fraction of potassium carbonate aqueous solution ( Potassium carbonate: 8.637g, 0.0625mol; water: 20.16g), in solvent toluene (19.5g), catalyst palladium acetate (0.0056g, 7.5*10 -5 mol) and ligand tricyclohexylphosphine (0.042g, 1.5*10 -4 mol) in the presence of the mixture, the temperature was raised to 55-60° C., and the reaction was complete for 2 hours (TLC followed the reaction). After the reaction, the mixture was cooled to 0-40°C, the aqueous phase was separated by liquid separation, the organic phase was washed with water to pH = 7, dried over anhydrous sodium sulfate, passed through a silica gel column, and concentrated by rotary evaporation to obtain a brownish yellow solid, which was weighed with toluene Crystallized to obtain a pale yellow solid, dried in vacuo to obtain 5...
Embodiment 3
[0072] 1) Preparation of Compound A
[0073]
[0074] Under nitrogen protection, 2-bromo-4-nitrobenzoic acid methyl ester (6.5g, 0.025mol), phenylboronic acid (3.2g, 0.0262mol) and 30% mass fraction of potassium carbonate aqueous solution ( Potassium carbonate: 8.637g, 0.0625mol; water: 20.16g), in solvent toluene (19.5g), catalyst palladium acetate (0.0056g, 7.5*10 -5 mol) and ligand tricyclohexylphosphine (0.042g, 1.5*10 -4 mol) in the presence of the mixture, the temperature was raised to 55-60° C., and the reaction was complete for 2 hours (TLC followed the reaction). After the reaction, the temperature of the mixture was lowered to 0-40°C, the aqueous phase was separated through liquid separation, the organic phase was washed with water to pH = 7, dried over anhydrous sodium sulfate, passed through a silica gel column, and concentrated by rotary evaporation to obtain a brownish-yellow solid, which was weighed with toluene Crystallized to obtain a pale yellow solid, d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com