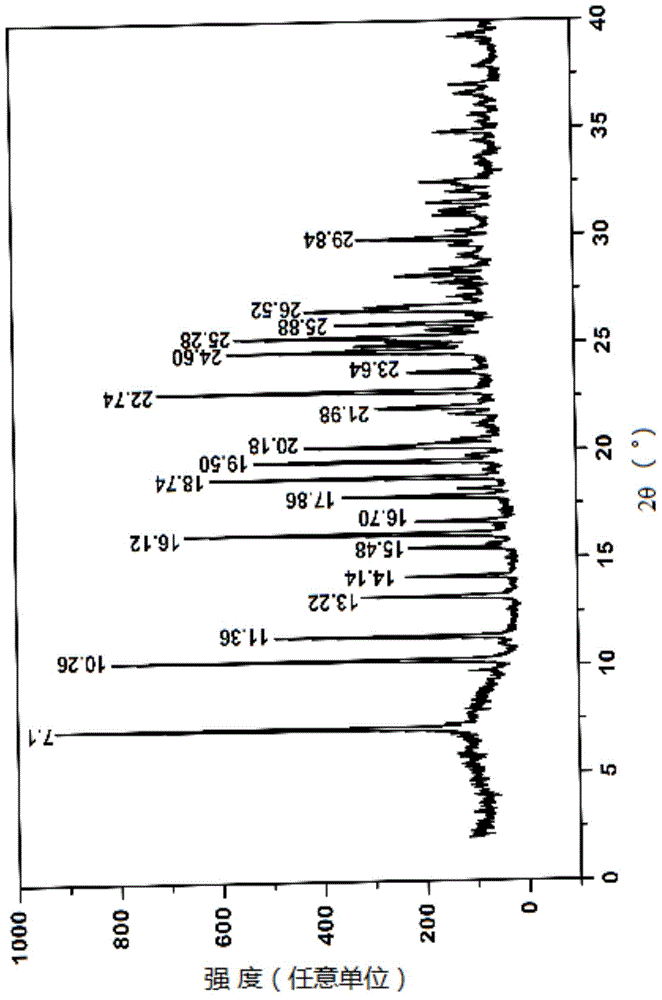
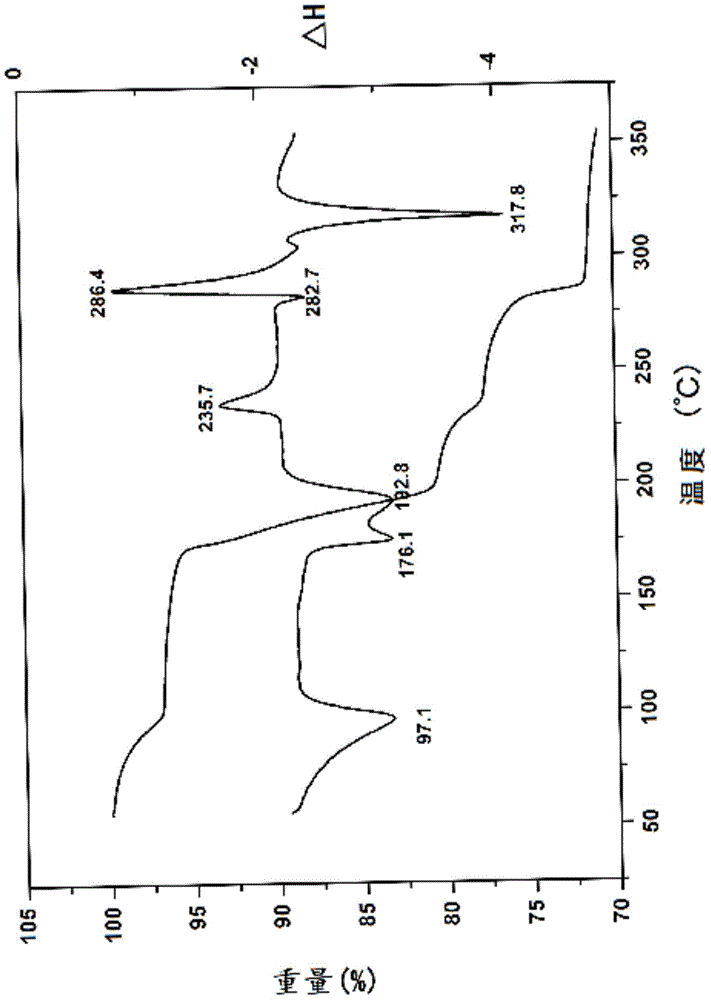
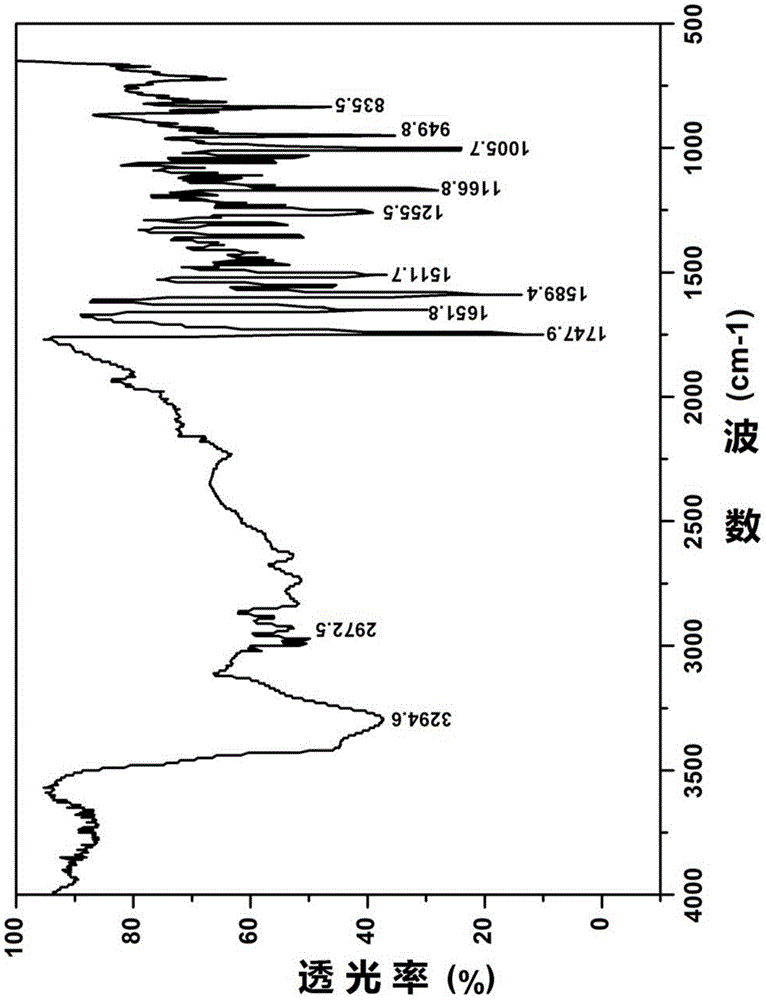
Novel 7-ethyl-10-hydroxycamptothecine crystal and preparation method thereof
A technology of hydroxycamptothecin and crystal form, which is applied in the field of medicinal chemistry, can solve the problem of low solubility of polymorphs, and achieve the effect of simple and easy-to-operate preparation method and high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, the preparation of 7-ethyl-10-hydroxycamptothecin crystal form
[0031] Weigh 50mg of 7-ethyl-10-hydroxycamptothecin into a reaction flask, add 1.5ml of dimethyl sulfoxide, stir to dissolve, evaporate the solvent at 20-30°C to crystallize, filter and separate the solid, and the solid is at 50°C Dry under vacuum until constant weight to obtain the crystal form of 7-ethyl-10-hydroxycamptothecin.
Embodiment 2
[0032] Embodiment 2, the preparation of 7-ethyl-10-hydroxycamptothecin crystal form
[0033] Weigh 30mg of 7-ethyl-10-hydroxycamptothecin into a reaction flask, add 1.5ml of dimethyl sulfoxide, stir to dissolve, the volatile solvent crystallizes at 20-30°C, filter and separate the solid, and the solid at 50°C Dry under vacuum until constant weight to obtain the crystal form of 7-ethyl-10-hydroxycamptothecin.
Embodiment 3
[0034] Embodiment 3, the preparation of 7-ethyl-10-hydroxycamptothecin crystal form
[0035] Weigh 30mg of 7-ethyl-10-hydroxycamptothecin into a reaction flask, add 3ml of N,N-dimethylformamide, stir to dissolve, add dropwise to 2ml of ethyl acetate solution, and crystallize from the volatile solvent at room temperature , the solid was separated by filtration, and the solid was vacuum-dried at 50° C. to constant weight to obtain the crystalline form of 7-ethyl-10-hydroxycamptothecin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com