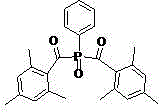
Preparation method of phenyl bis(2,4,6-trimethylbenzoyl)phosphine oxide
A technology of trimethylbenzoyl and phenylbis, which is applied in the field of preparation of high-efficiency free radical photoinitiator phenylbisphosphine oxide, can solve the problems of high environmental pressure, difficult industrial implementation, and high cost, and achieves no environmental pressure. , Improve the reaction rate, the effect of cleaning by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the preparation of phenylphosphine
[0043] Add 500ml of toluene and sodium block (10.1g, 0.88mol) into a 1000ml reaction bottle under the protection of argon, heat until reflux, stir vigorously, and slowly add dichlorobenzene dropwise when the sodium block becomes very fine Phenylphosphine (39.4g, 0.22mol), after 0.5h of dripping, continue to keep warm for 16h, cool down to below the boiling point, then slowly add water (10.0g, 0.55mol), reflux reaction until the sodium is completely consumed, and the phenylphosphine containing solution.
[0044] Low-pressure distillation recovered the solvent for purification, and collected 40-45°C / 10 mHg fractions to obtain 20.0 g of phenylphosphine, which was confirmed by nuclear magnetic resonance. 31 P: -123ppm.
[0045] Or install a water separator, reflux for dehydration, and remove 50ml of toluene-water mixture to obtain anhydrous toluene solution of phenylphosphine, which is confirmed by nuclear magnetic reson...
Embodiment 2
[0046] Embodiment 2: Preparation of phenyl bis (2,4,6-trimethylbenzoyl) phosphine oxide
[0047] Under the protection of argon, take 17.3g of sodium hydride (60%, 0.44mol) and evenly disperse in 50ml of toluene, slowly add dropwise 20.0g (0.18mol) of phenylphosphine (dissolved in 450ml of toluene solution) prepared in Example 1 After dropping, reflux reaction for 2-4h, the color of the reaction system changed from yellow-green to dark yellow, and finally into a reddish-brown viscous system, down to room temperature, dropwise added 73.1g (0.40mol) 2,4,6-trimethyl Benzoyl chloride, the reaction is exothermic, after dripping, the reaction system gradually turns into a red transparent solution, an orange transparent solution, the temperature is raised to about 40°C, and the reaction is kept for about 5 hours. The reaction is monitored by liquid chromatography. After the reaction is complete, add hydrochloric acid solution to wash , adjust the pH at 4-6, stir for 30min, stand and...
Embodiment 3
[0048] Embodiment 3: Preparation of phenyl bis (2,4,6-trimethylbenzoyl) phosphine oxide
[0049] Under the protection of argon, get 17.3g sodium hydride (60%, 0.44mol) and evenly disperse in 50ml toluene, slowly add the anhydrous toluene solution of the phenylphosphine prepared after embodiment 1 divides water (from dichlorobenzene phenylphosphine (39.4g, 0.22mol) prepared phenylphosphine solution), dropwise, reflux reaction for 2-4h, the color of the reaction system changed from yellow-green to dark yellow, and finally into a reddish-brown viscous system, down to room temperature , add 80.4g of 2,4,6-trimethylbenzoyl chloride dropwise, the reaction is exothermic, after dropping, the reaction system gradually turns into a red transparent solution, an orange transparent solution, the temperature is raised to about 40°C, and the reaction is kept for about 5 hours. Monitor the reaction by phase chromatography. After the reaction is complete, add 120ml of 5% hydrochloric acid so...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap