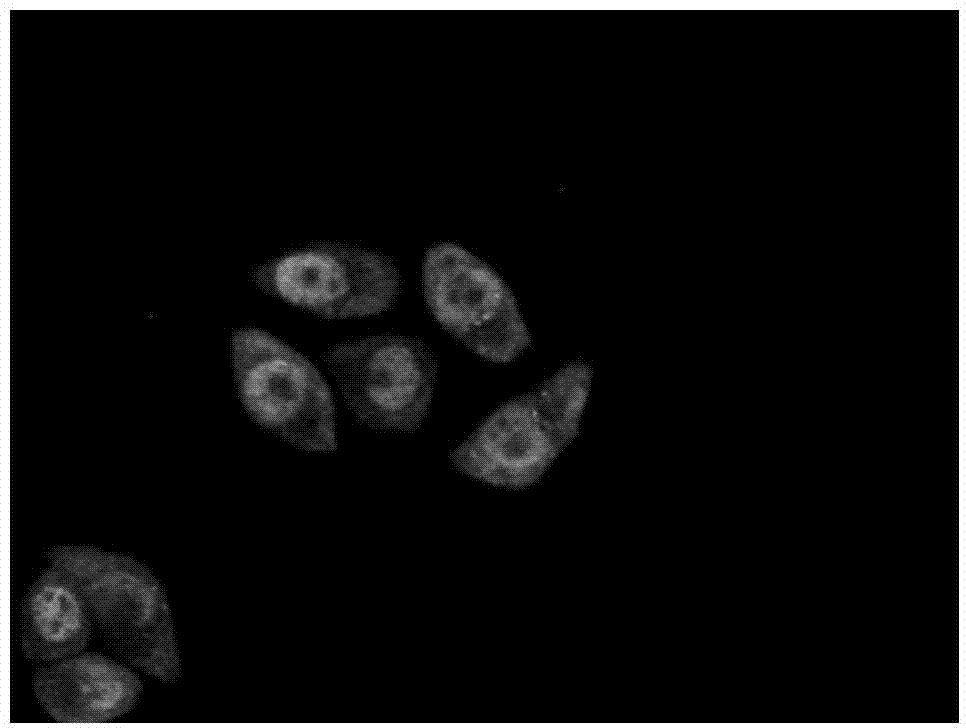
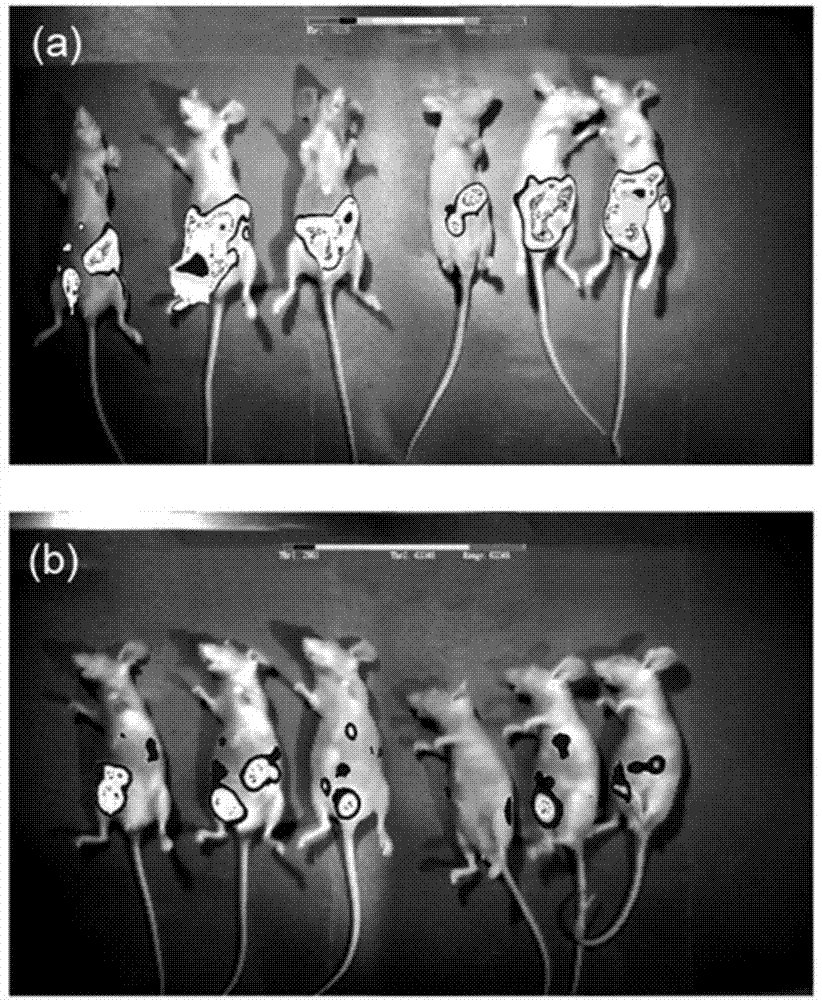
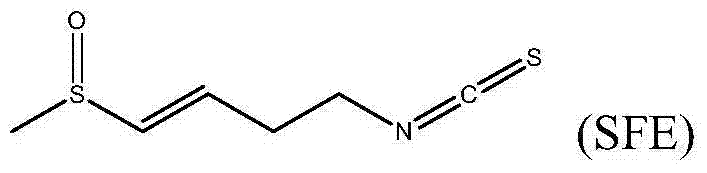
XPO1 inhibitor
A protein inhibitor, 1.XPO1 technology, applied in anti-inflammatory agents, antiviral agents, non-central analgesics and other directions, can solve the problems of toxic side effects, limited relief of inflammatory bowel disease, etc., to achieve small side effects, biological The effect of good safety and bioavailability, huge market space and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1. Preparation of compound F-01
[0075]
[0076] Step 1. Preparation of (2-ethyl)-1-propylthiophenol-ethyl methyl phosphate
[0077]
[0078] Dissolve 0.76 g of 1-propylthiophenol and 1.38 g of potassium carbonate in 50 ml of DMF solution, slowly add 1.50 g of diethyl methyl methanesulfonate phosphate dropwise at room temperature, stir at room temperature, detect by TLC, for 2 hours After the raw material is no longer reduced, the reaction is complete. The combined organic layers were extracted and dried, and 0.71 g of yellow oily liquid (yield rate was 72%) was obtained by column chromatography. The mass spectrum MS of the compound: [M+H] + 226.1.
[0079] Step 2. Preparation of (2-Ethyl)-1-Propylthiophenol Sulphinyl-Methyl Phosphate Ethyl Ester
[0080]
[0081]2.26 g of the product from step 1 was dissolved in 15 ml of chloroform solution, and then 0.25 g of m-CPBA solution was slowly added dropwise. Stir at room temperature and detect by TLC...
Embodiment 2
[0085] Embodiment 2. Preparation of compound F-03
[0086]
[0087] Step 1. Preparation of (2-ethyl)-1-cyclopropylthiophenol-ethyl methyl phosphate
[0088]
[0089] Dissolve 0.74 g of 1-cyclopropylthiophenol and 1.38 g of potassium carbonate in 50 ml of DMF solution, slowly add 1.50 g of diethyl methyl methanesulfonate phosphate dropwise at room temperature, stir at room temperature, and detect with TLC, 2 After 1 hour, the raw materials no longer decrease, and the reaction is completed. The combined organic layers were extracted and dried, and 1.81 grams of yellow oily liquid (yield rate 85%) were obtained by column chromatography. The mass spectrum MS of the compound: [M+H] + 224.2.
[0090] Step 2. Preparation of (2-Ethyl)-1-Propylthiophenol Sulphinyl-Methyl Phosphate Ethyl Ester
[0091]
[0092] 2.24 g of the product from step 1 was dissolved in 20 ml of chloroform solution, and then 0.25 g of m-CPBA solution was slowly added dropwise. Stir at room temperatu...
Embodiment 3
[0096] Embodiment 3. Preparation of compound F-05
[0097]
[0098] Step 1. Preparation of (2-ethyl)-methylthiophenol-ethyl methyl phosphate
[0099]
[0100] Dissolve 0.50 g of methylthiophenol and 1.38 g of potassium carbonate in 50 ml of DMF solution, slowly add 1.50 g of diethyl methyl methanesulfonate phosphate dropwise at room temperature, stir at room temperature, and detect by TLC. After 2 hours, the raw material no longer decrease, the reaction is complete. The combined organic layers were extracted and dried, and 1.32 grams of yellow oily liquid (yield rate 72%) were obtained by column chromatography. The mass spectrum MS of the compound: [M+H] + 198.2.
[0101] Step 2. Preparation of (2-ethyl)-1-methylthiophenol sulfinyl-methylphosphonate
[0102]
[0103] 1.99 g of the product from step 1 was dissolved in 25 ml of chloroform solution, and then 0.80 g of m-CPBA solution was slowly added dropwise. Stir at room temperature and detect by TLC. After 2 hours...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com