Immunoassay method and kit for detecting concentration of cyclosporine A
A cyclosporine and immunoassay technology, applied in the field of medical testing, can solve the problem of reducing the ability of B cells to produce high-affinity antibodies, reducing the ability of T lymphocytes to secrete lymphokines, and weakening the antigen presentation of T lymphocytes, etc. To achieve the effect of improving the detection sensitivity of CsA, improving the detection sensitivity and high practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079] The preparation method of described cyclosporine A immunogen comprises the steps:
[0080] A) Under the irradiation of a 300W iodine-gallium lamp, the -OH of CsA reacts photochemically with BBa to generate CsA-BBa with a carboxyl group;
[0081] B) Coupling activated CsA-BBa with carrier protein by EDCI or EDCI-NHS method to form immunogenic CsA-protein complex.
[0082] Wherein, the sample pretreatment reagent includes a whole blood lysing agent and a whole blood sedimentation agent, which function to lyse blood cells and release protein-bound CsA. The whole blood dissolving agent is composed of a surfactant; the whole blood sedimentation agent is composed of an organic solvent containing divalent metal ions, or one or more of protein denaturants such as urea, guanidine hydrochloride, and proteolytic enzymes .
[0083] Wherein, described calibrator refers to the homogeneous whole blood solution that contains known CsA concentration, in order to sample CsA concentrati...
Embodiment 1
[0087] Embodiment 1 coating uses the preparation of CsC-BSA protein complex
[0088] 1.1 Carboxylated cyclosporine C
[0089] Dissolve 35 mg of cyclosporine C in 1 ml of dichloromethane, add 12 mg of succinic anhydride, 5.9 mg of 4-dimethylaminopyridine, and 8 μl of triethylamine, and stir at room temperature for 24 hours. Vacuum dry under negative pressure, remove the organic solvent, and dissolve the residue with 3 ml of dichloromethane. Wash twice with 0.1M dilute hydrochloric acid, collect the organic phase; wash once with saturated NaCl solution. Pipette the organic phase into the reagent bottle, fully dry it with anhydrous sodium sulfate, recover the organic phase, and spin dry under reduced pressure to obtain off-white carboxylated cyclosporine C (cyclosporin C-semisuccinic acid) of about 33.7 mg.
[0090] The LC-MS mass spectrum of cyclosporine C after carboxylation is as follows image 3 as shown, image 3 The most abundant peak in the middle corresponds to the t...
Embodiment 2
[0095]Embodiment 2 is coated with the preparation of CsA-BSA protein complex
[0096] 2.1 Carboxylated cyclosporine A
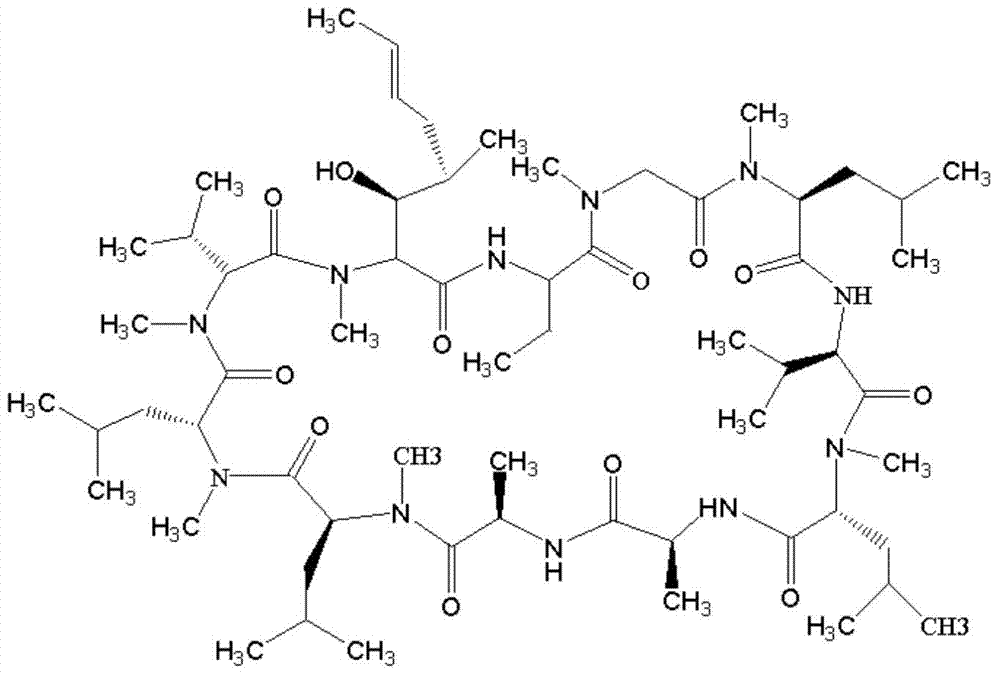
[0097] Add 60 mg of CsA and 12 mg of 4-benzoylbenzoic acid (BBa) into a 15 ml quartz test tube, dissolve in 6 ml of acetone, and continue to bubble nitrogen gas for 20 minutes. Turn on the iodine-gallium lamp tube (300W, the lamp tube is 10cm away from the test tube), and react for 8 hours under magnetic stirring and nitrogen atmosphere. Using chloroform: methanol (volume ratio 85:15) as the developing solvent, the CsA-BBa product was separated and purified by chromatography on a preparative silica gel plate. Add 3ml of DMF to wash, filter, take the organic phase and spin dry under negative pressure to obtain 23.5mg of light gray solid.
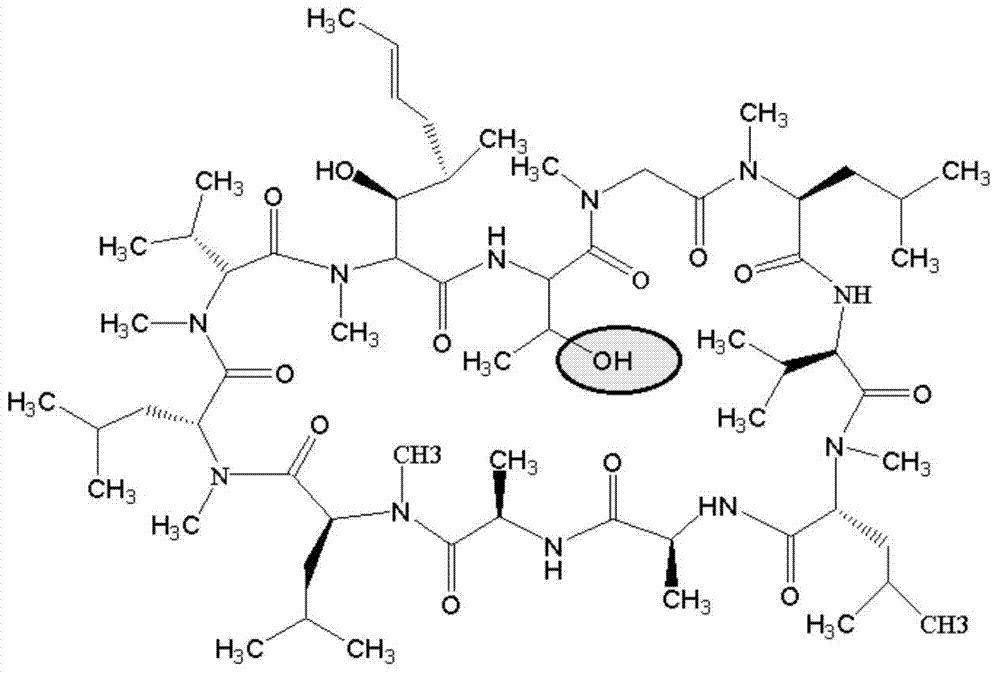
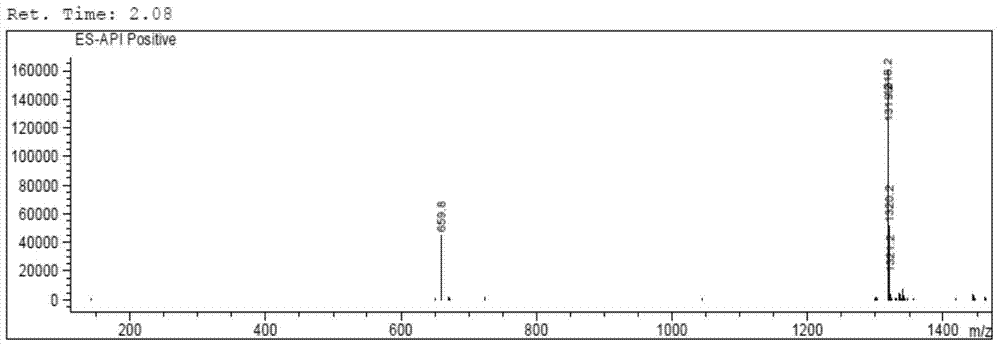
[0098] The LC-MS mass spectrum of cyclosporine A after carboxylation is as follows Figure 4 as shown, Figure 4 The peak with the largest abundance in the medium corresponds to the target product CsA-BBa, with a molecul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com