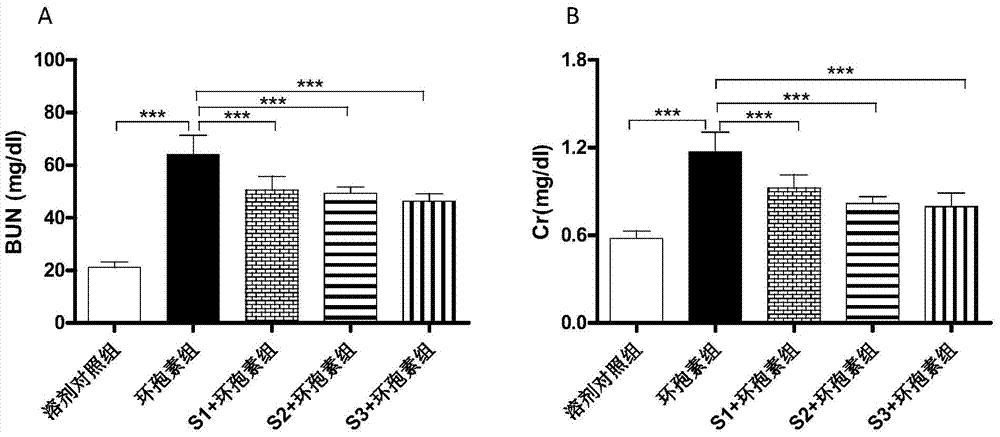
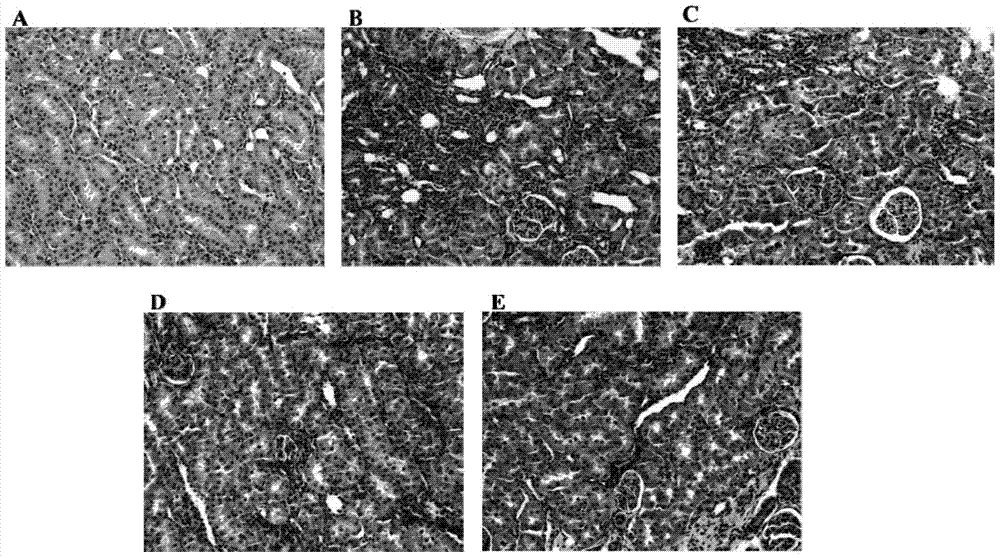
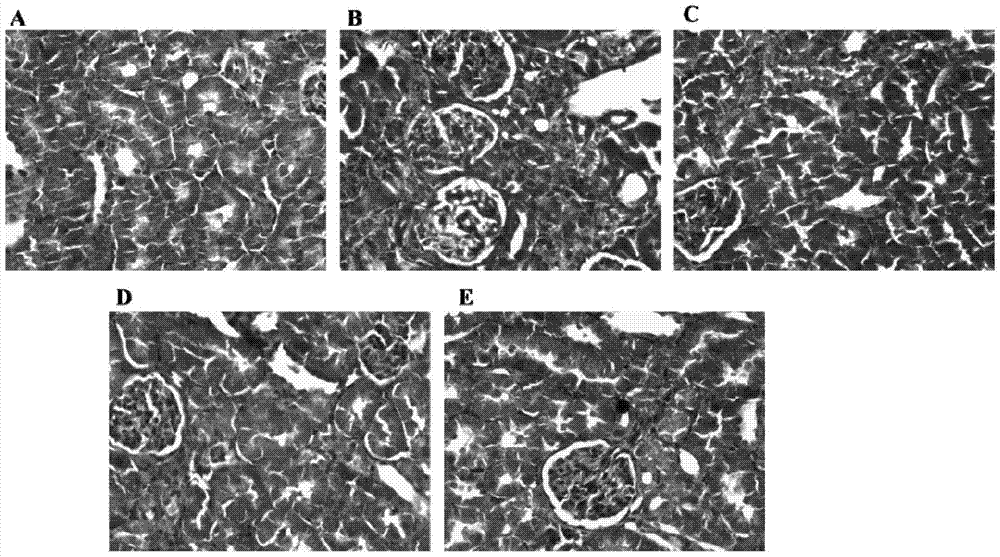
A pharmaceutical composition for preventing and treating cyclosporin A chronic nephrotoxicity
A composition, nephrotoxicity technology, applied in the direction of drug combination, pharmaceutical formula, plant raw materials, etc., can solve the problems of difficulty, complex occurrence mechanism, retention, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0112] Example 1: (granules)
[0113] Prescription: 1200g of golden cherry, 1000g of emblica, 600g of schisandra, 1500g of gynostemma
[0114] Preparation method: Extract the raw material medicine with water for 3 times according to the above ratio, add 12 times of water to decoct for 1 hour each time, then combine the decoction liquid, filter, and finally concentrate the filtrate to an extract with a relative density of about 1.30, and then decoct it by weight Take a mixture of dextrin and mannitol that is twice the amount of the extract, the ratio of dextrin and mannitol is 1:1.5, mix the two thoroughly, then add a small amount of extract to make a soft material, and pass through a 24-mesh sieve Granulated, dried, whole, divided into packs of 15 grams.
[0115] Dosage: 15g each time, 2 times a day, take it with warm water.
example 2
[0116] Example 2: (granules)
[0117] Prescription: 1200g golden cherry, 900g emblica, 600g schisandra, 1600g gynostemma
[0118] Preparation method: Extract the raw material medicine with water for 3 times according to the above ratio, add 12 times of water to decoct for 1 hour each time, then combine the decoction liquid, filter, and finally concentrate the filtrate to an extract with a relative density of about 1.30, and then decoct it by weight Take a mixture of dextrin and mannitol that is twice the amount of the extract, the ratio of dextrin and mannitol is 1:1.5, mix the two thoroughly, then add a small amount of extract to make a soft material, and pass through a 24-mesh sieve Granulated, dried, whole, divided into packs of 15 grams.
[0119] Dosage: 15g each time, 2 times a day, take it with warm water.
example 3
[0120] Example 3: (granules)
[0121] Prescription: 1300g golden cherry, 900g emblica, 600g schisandra, 1500g gynostemma
[0122] Preparation method: Extract the raw material medicine with water for 3 times according to the above ratio, add 12 times of water to decoct for 1 hour each time, then combine the decoction liquid, filter, and finally concentrate the filtrate to an extract with a relative density of about 1.30, and then decoct it by weight Take a mixture of dextrin and mannitol that is twice the amount of the extract, the ratio of dextrin and mannitol is 1:1.5, mix the two thoroughly, then add a small amount of extract to make a soft material, and pass through a 24-mesh sieve Granulated, dried, whole, divided into packs of 15 grams.
[0123] Dosage: 15g each time, 2 times a day, take it with warm water.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com