Alkaline thermal-stability SGNH family esterase EstD1 and gene thereof
A thermostable, family-friendly technology, applied in the field of genetic engineering, which can solve the problems of difficult purification, long fermentation period of natural strains, and difficulty in mass production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: Cloning of SGNH family esterase gene EstD1
[0046] Extract the genomic DNA of thermophilic Alicyclobacillus D-1: CTAB lysis method, the specific steps are: centrifuge the fresh bacterial liquid cultured in the liquid for 12 hours, and add 800 μL of solution Ⅰ (20mM Tris, pH8.0, 2mM Na 2 -EDTA, final concentration 20mg / mL lysozyme), incubate at 37°C for 30min, add 100μL 10% SDS, mix up and down, add 10μL 10mg / mL proteinase K, keep at 37°C for 30min, add 150μL 5M NaCl and 150μL 10% CTAB solution was mixed upside down, kept at 65°C for 20min, divided into 600μL tubes, then added 600μL of phenol / chloroform / isopropanol (volume ratio 25:24:1) for extraction, and centrifuged at 12000rpm at 4°C for 10min. Take 300 μL of the supernatant and extract it again in 300 μL of chloroform / isopropanol (volume ratio 24:1) to remove impurities, centrifuge at 12000 rpm for 10 min at 4°C, then take the supernatant and add 2 times the volume of absolute ethanol and 1 / 10 volume ...
Embodiment 2
[0053] Embodiment 2: Preparation of recombinant SGNH family esterase EstD1
[0054] Take the recombinant plasmid pEASY TM - BL21(DE3) strain of E2-EstD1 and pEASY only TM -The empty plasmid BL21(DE3) strain of E2 was inoculated in LB (containing 100 μg / mL Amp r ) culture medium, shake rapidly at 37°C for 16h. Then this activated bacterial solution was inoculated into fresh LB (containing 100 μg / mL Amp r ) medium, cultured with rapid shaking for about 2–3 hours (OD 600 After reaching 0.4-0.6), add IPTG with a final concentration of 0.7mM for induction, and continue shaking culture at 20°C for about 20h. Centrifuge at 12000rpm for 10min to collect the bacteria. With an appropriate amount of pH7.0 citric acid-Na 2 HPO 4 After suspending the bacteria in the buffer solution, the bacteria were disrupted by ultrasonic in a low-temperature water bath. The crude enzyme solution concentrated in the above cells was centrifuged at 12,000rpm for 10min, the supernatant was aspirated...
Embodiment 3
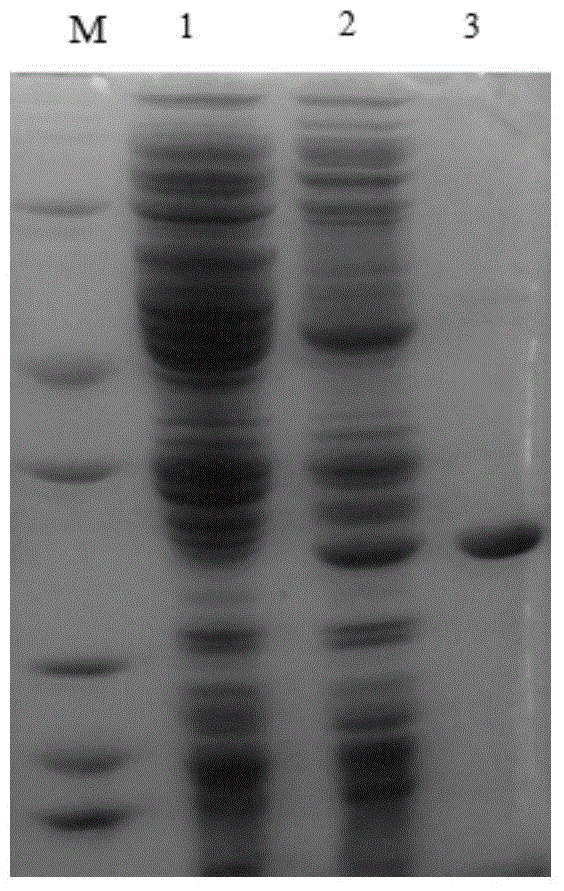
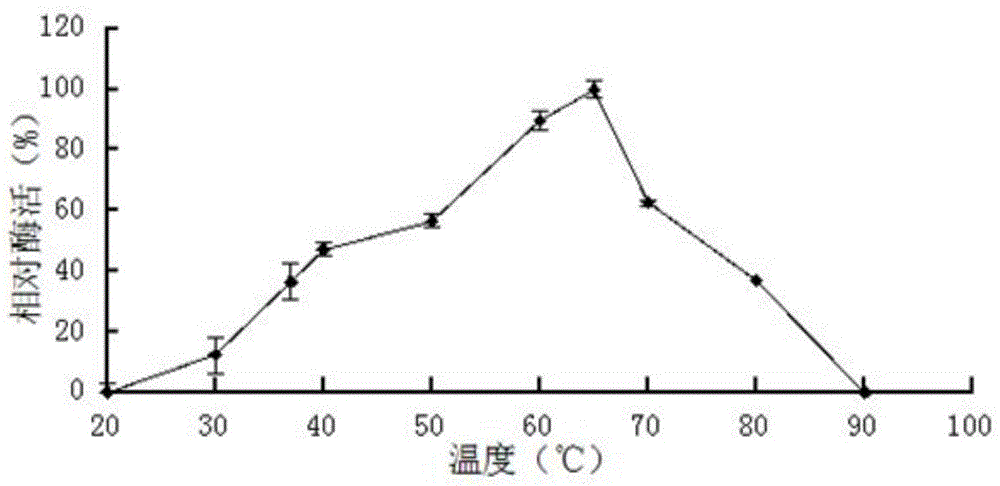
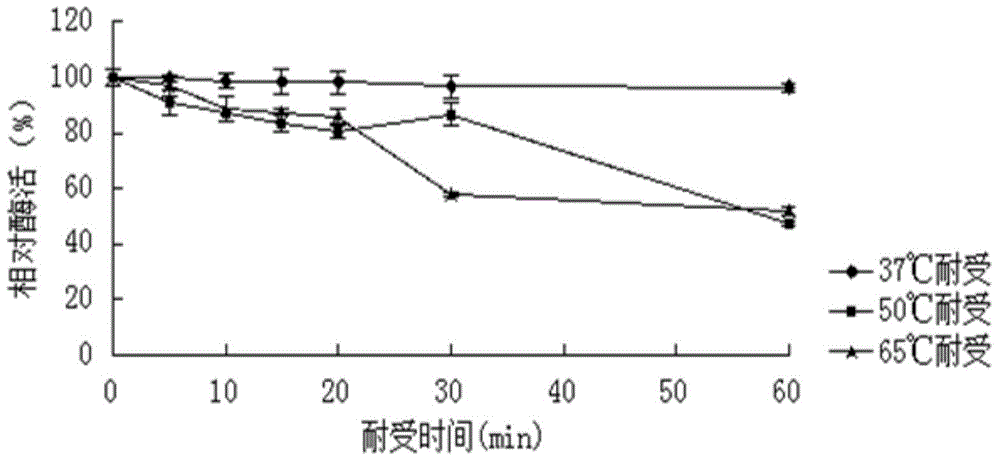
[0055] Example 3: Characterization of the purified recombinant SGNH family esterase EstD1
[0056] (1) Activity analysis of recombinant SGNH family esterase EstD1
[0057] The activity of the purified recombinant SGNH family esterase EstD1 was determined by the p-nitrophenol method: take four 1.5mL centrifuge tubes, numbered 1, 2, 3, and 4 respectively, and the test tubes 1, 2, and 3 are Experimental group (3 repetitions), No. 4 is the control group. Add 420 μL of 50 mM Tris-HCL buffer at pH 8.0 and 30 μL of 10 mM substrate pNPC to four centrifuge tubes respectively 2 , after preheating at 37°C for 5 minutes, add 50 μL enzyme solution of appropriate dilution to tubes 1, 2, and 3 every 10 seconds, add the same amount of water to control tube 4, react at 37°C for 5 minutes, and add to tubes 1 and 3 every 10 seconds. 2. Add 50μL 0.1M Na to the experimental tube 2 CO 3 To terminate the reaction, add an equal volume of stop solution to the No. 4 control tube and immediately ins...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com