Application of 17-allylamine-17-demethoxygeldanamycin in preparation of medicine for treating epilepsy
A technology of methoxygeldanamycin and allylamine, which is applied in the field of 17-propenamine-17-desmethoxygeldanamycin to prepare medicines for the treatment of refractory epilepsy, and can solve the problem of no epilepsy reports, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The establishment of embodiment 1 epilepsy mouse model
[0038] 1. Drug injection method: Reagent:
[0039] DMSO: Dimethyl sulfoxide (Sigma, D4540-1L Lot#BCBK5723V)
[0040] 17-Allylamino-17-Demethoxygeldanamycin: 17AAG, [17-(Allylamino)-17-demethoxygeldanamycin], [17-(Allylamino)geldanamycin], [Tanespimycin] (LC Laboratories, A-688017- AAG, >99%,), Geldanamycin: (LC Laboratories, G-4500)
[0041] Preparation method:
[0042] (1) Dilute 17-propenylamine-17-desmethoxygeldanamycin to 50 mg / ml with DMSO, aliquot and store at -20°C in the dark.
[0043] (2) Injection concentration: 20 mg (diluted with DMSO, the final volume of each injection per mouse is 50 μl).
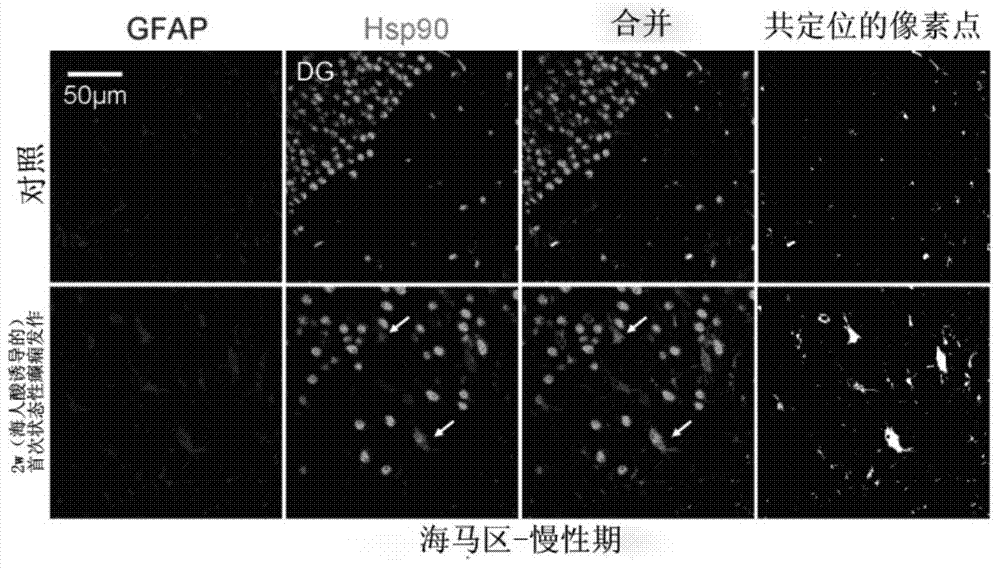
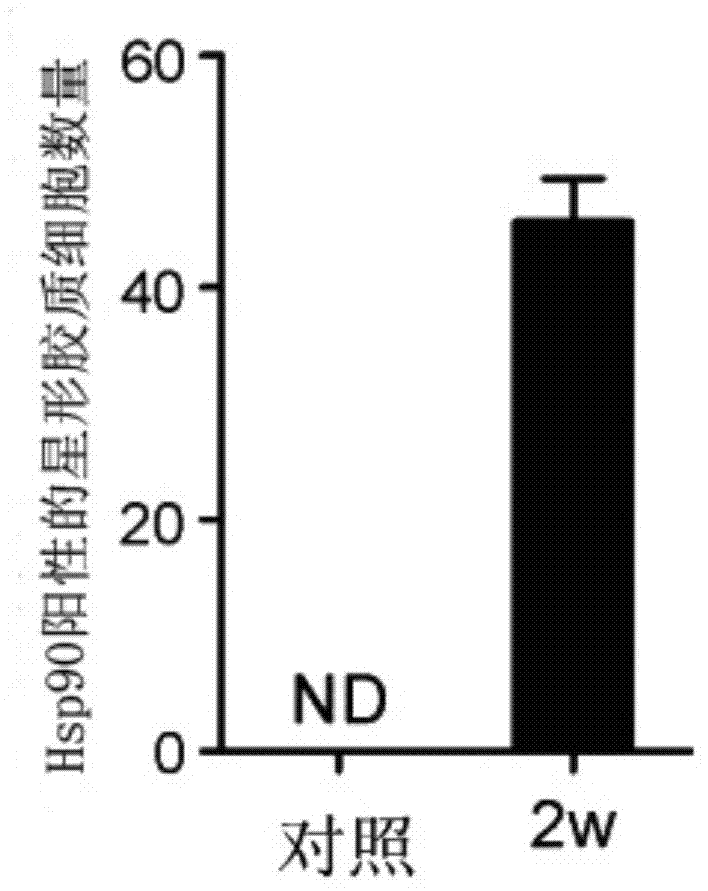
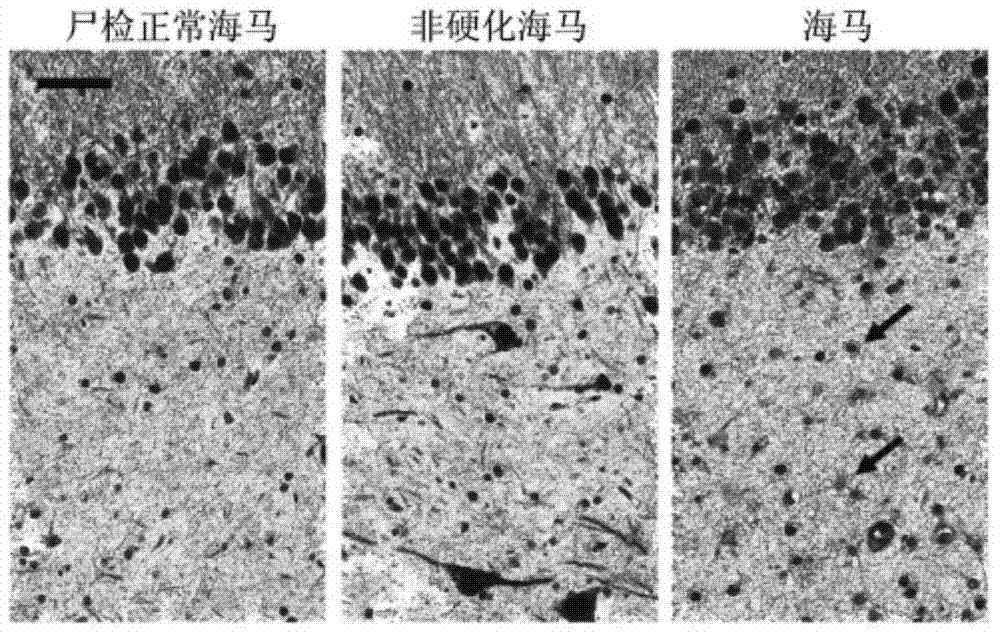
[0044] 2. Establishment method of model mice: We use unilateral hippocampus injection of kainate-induced medial temporal lobe epilepsy model mice, which is the classic and most commonly used model of refractory temporal lobe epilepsy. The first seizure lasting about 3 hours was induced by unilateral injection...
Embodiment 2
[0045] Example 2 Effects of Drugs on the Expression Level of Mouse Hippocampus Glt1
[0046] 1. Experimental method
[0047] First, normal mice were intraperitoneally injected with 17-propenylamine-17-desmethoxygeldanamycin to determine the optimal injection dose and injection time. We selected a total of 5 experimental doses: 0mg / kg, 2mg / kg, 5mg / kg, 15mg / kg, 25mg / kg, and took mouse hippocampal protein at 24 hours, and found that when the injection concentration was 15mg / kg, monomer Glt1 The up-regulation of Glt1 is the most obvious, which is about 2.8 times that of the control. When the injection concentration is 25 mg / kg, although the expression level of monomeric Glt1 is decreased relative to the injection concentration of 15 mg / kg, the expression level of tetrameric Glt1 is increased. More obvious, about 2.0 times of the control (Figure 2). In the follow-up experiments, the injection concentration we chose was 20mg / kg.
[0048] For the selection of optimal injection tim...
Embodiment 317
[0051] Example 31 Therapeutic Effect of 7-Allylamine-17-Demethoxygeldanamycin on Mouse Model Epilepsy
[0052] 1. Experimental method
[0053] After the baseline measurement, 17-propenylamine-17-desmethoxygeldanamycin or DMSO was administered. The dosing cycle is as follows: firstly, 2 rounds of 6-day dosing are carried out, in which the drug is injected continuously for the first five days, and no medicine is given on the sixth day. Then inject the drug every other day ( image 3 ). In this experiment, 13 mice were injected with 17-propenylamine-17-desmethoxygeldanamycin, and 8 mice were injected with DMSO. The results of EEG monitoring showed that the injection of DMSO itself did not affect the frequency of seizures before and after injection, while the injection of 17-propenylamine-17-desmethoxygeldanamycin significantly inhibited the number of seizures and effectively relieved the seizures ( Figure 4 , histogram, the left column of groups 1-21 is the baseline, the gro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com