Ergothioneine production through metabolic engineering
An ergothioneine and gene technology, applied in the field of ergothioneine production through metabolic engineering, can solve the problems that EgtE enzyme cannot be overexpressed, EgtB activity is low, and production is limited.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
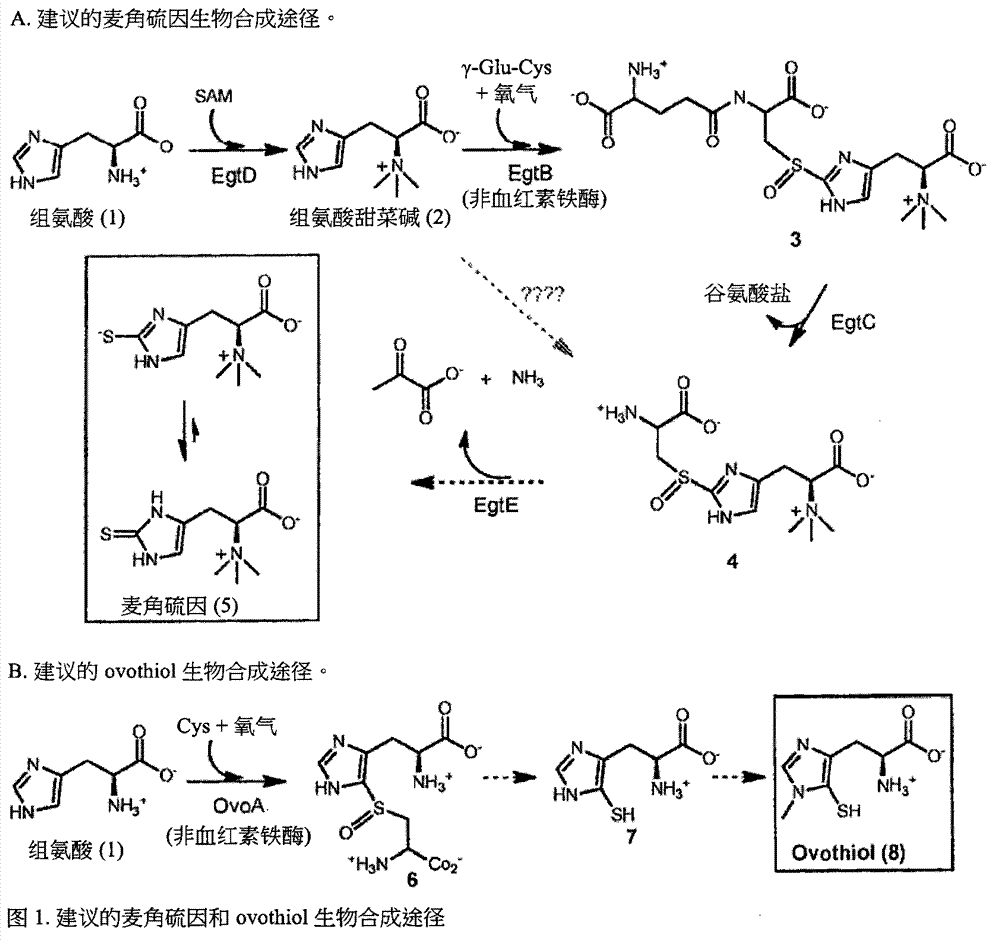
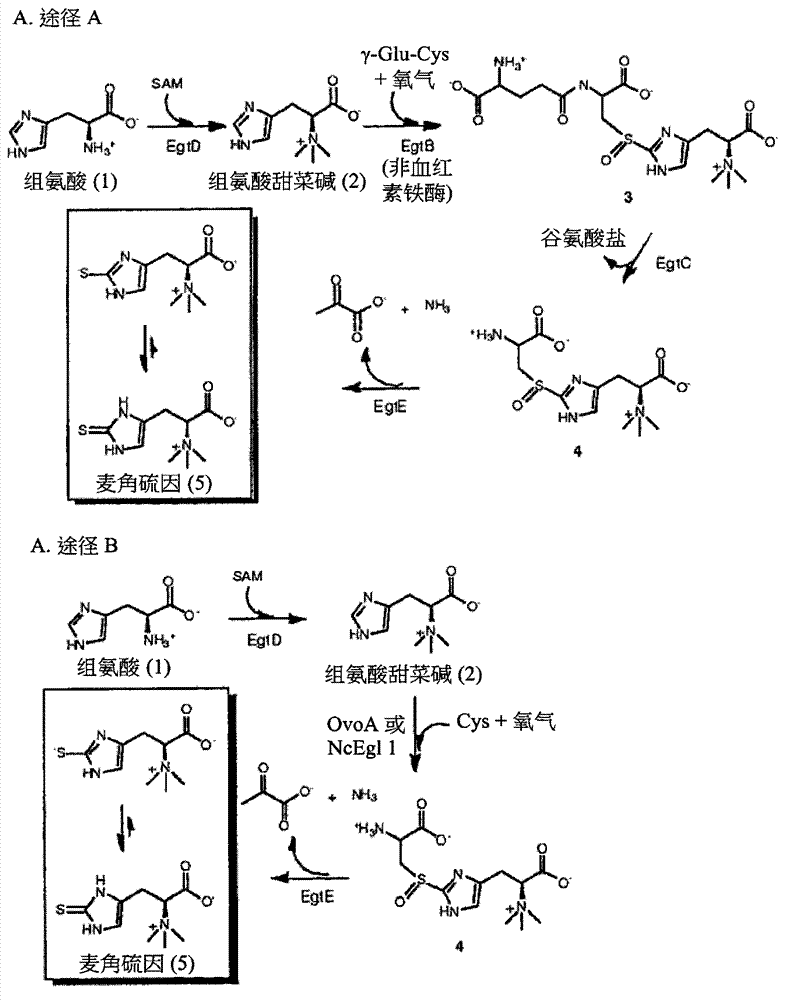
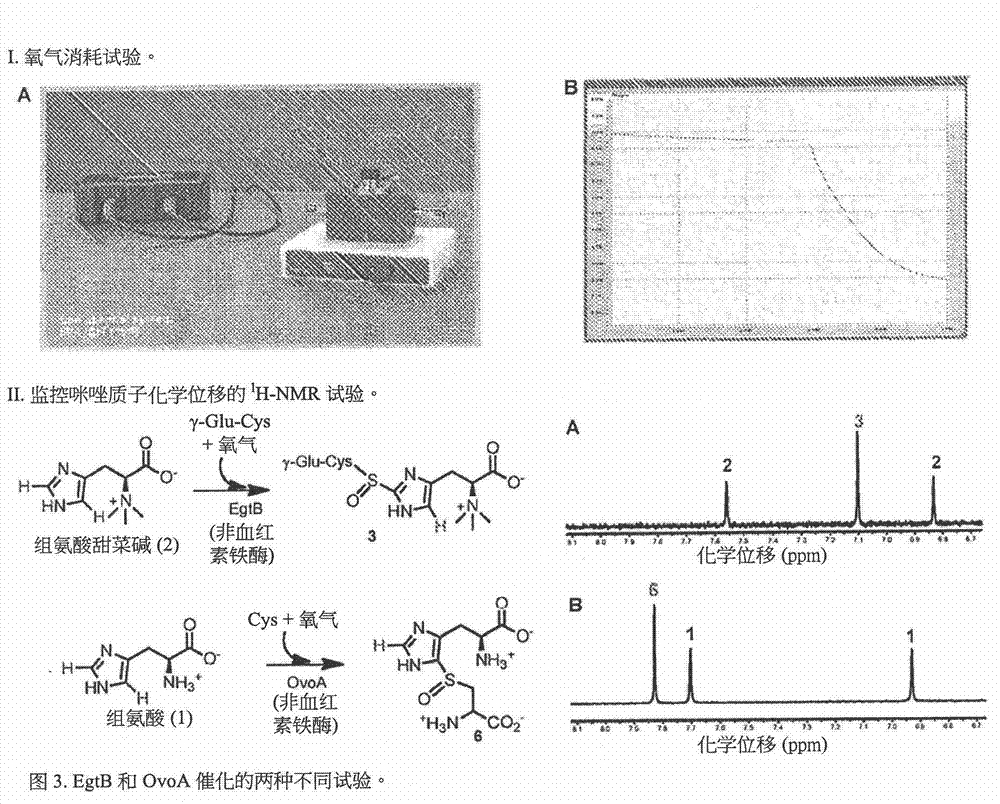
[0307] Example 1: Production of Ergothioneine by Metabolic Engineering (In Vitro Enzymatic Means)
[0308] Identification of a gene cluster for ergothioneine biosynthesis. Ergothioneine and ovothiol (5&7, figure 1 ) are metabolites of two thiol-imidazoles isolated from ergot by Tanret in 1909 [1]. The presence of ergothioneine-specific transporters in humans [2] allows us to enrich ergothioneine from the diet, reaching millimolar concentrations in several organs such as the liver, kidney, central nervous system and erythrocytes[ 3]. Unlike other thiols, the balance between the thiolate and thione forms of the thiol-imidazole side chain in ergothioneine and ovothiol is predominantly in favor of the thione form (e.g. figure 1 5) in [3f, 4], which makes them more stable in oxidation than other thiol mimics (eg, glutathione) [4a]. This unique redox property of ergothioneine [4a, 5] enables it to protect hemoglobin from oxidation in erythrocytes and avoid cataract formation in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com