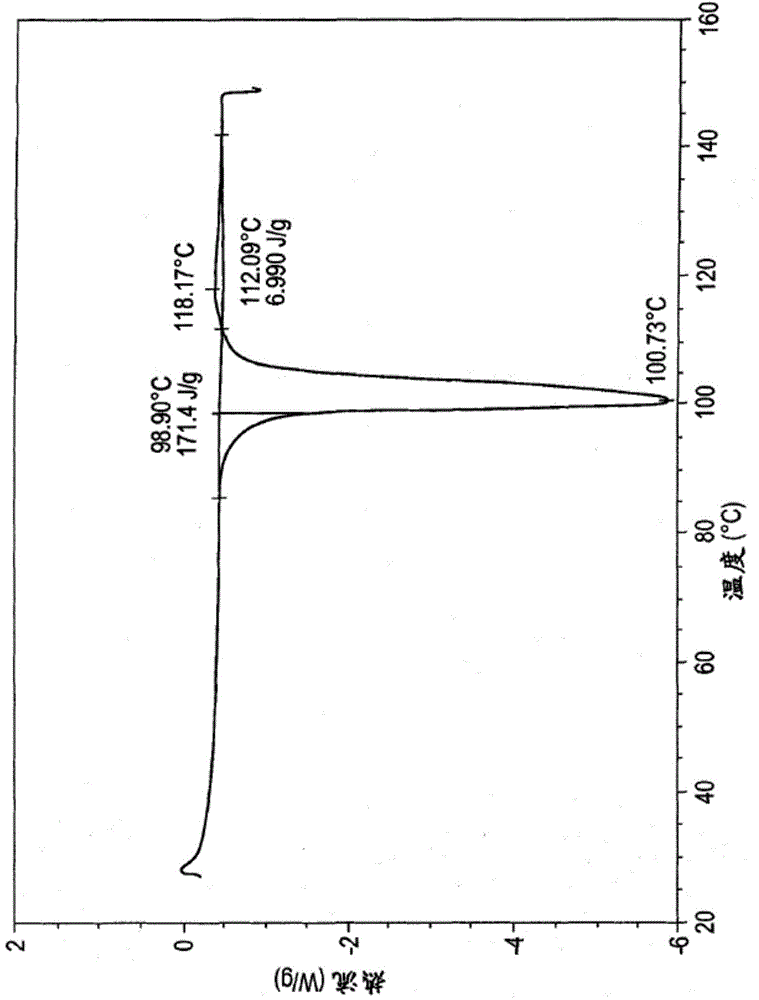
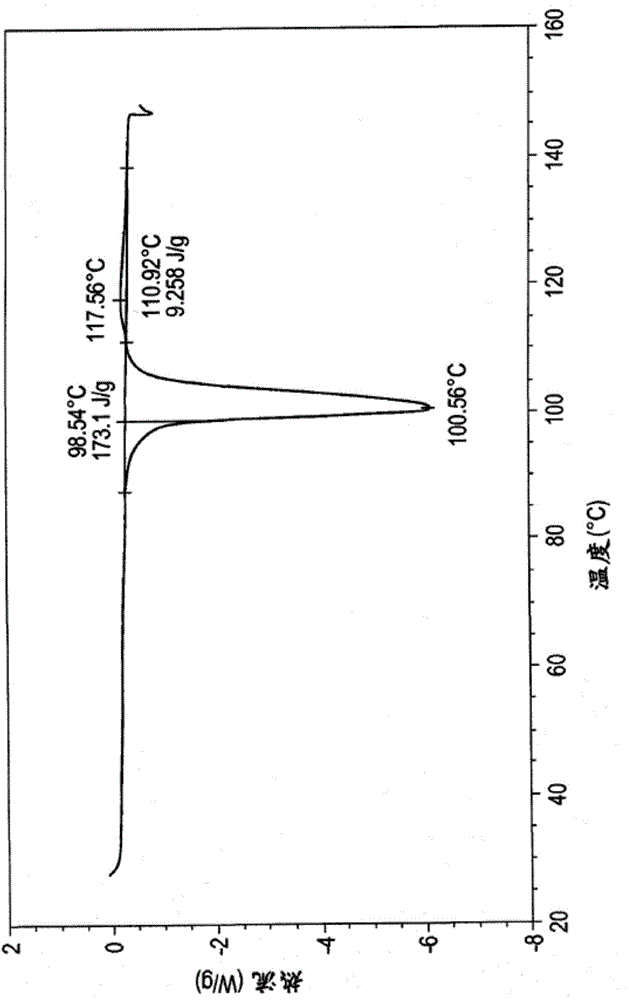
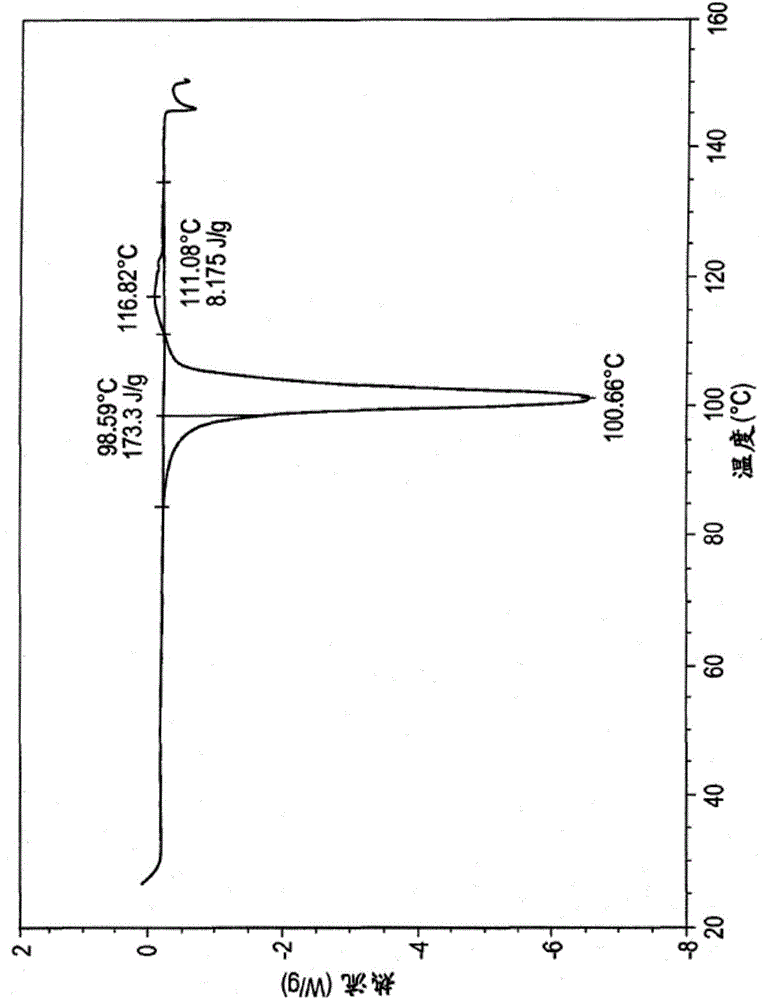
High content sodium ibuprofen granules, their preparation and their use in preparing non-effervescent solid dosage forms
A technology of ibuprofen sodium and granules, which is applied in the field of high-content ibuprofen pharmaceutical granules, and can solve the problem of large tablets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] Preparation of Granules of the Invention
[0058] To prepare the granules of the present invention, the above three main components are mixed in a suitable ratio to each other in a high shear granulator while wetting with water. In actual preparation, it is desirable to form a solution of polyvinylpyrrolidone in water before forming the overall mixture of the three components. This results in a more uniform distribution of the polyvinylpyrrolidone in the granules. After blending and granulation in a high shear granulator, water is removed from the wet granules by conventional drying procedures such as pan drying in an oven or fluid bed drying. Typically, drying is carried out at temperatures up to about 100°C. Preferably the oven temperature for drying the granules is in the range of about 60 to about 70°C. In the case of fluid bed dryers, the inlet temperature is also preferably in the range of about 60 to about 70°C. In a particularly preferred drying operation,...
example 1
[0084] Granule preparation
[0085] Prepared by the wet granulation method of the present invention is composed of 95.5% by weight of sodium ibuprofen hydrate, 2% by weight of sodium carbonate and 2.5% by weight of polyvinylpyrrolidone (Plasdone K-90, International Specialty Products Inc., Wayne, NJ). composed of particles. The method employed involved dissolving Plasdone K-90 in water, adding the solution to a V-blender equipped with a high shear intensifier drive (MAXI-BLENDLAB V-BLENDER, manufactured by GlobePharma, Inc., New Brunswick, NJ ) and form granules by operating the mixer using high shear, adding sieved sodium carbonate powder to the wet mixture in the mixer and mixing for an additional 5 minutes using the intensifier drive. After draining the contents of the mixer into a pan, the granules were dried in an oven maintained at 50°C until all added water was removed. The product was then screened through a stainless steel No. 16 mesh US Standard sieve.
example 2
[0087] Tablet preparation
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average particle size | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com