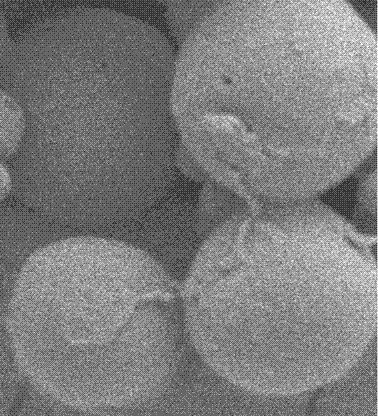
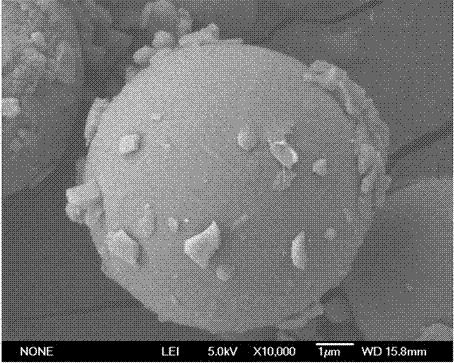
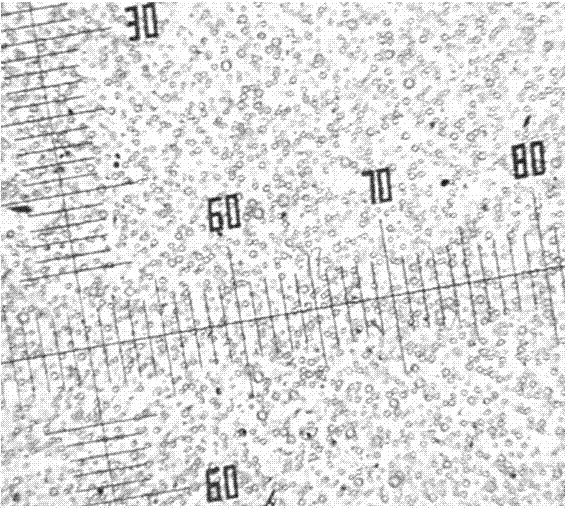
A kind of biodegradable emamectin benzoate microspheres
A technology of methylamino abamectin and benzoate, applied in the field of pesticides, can solve the problems of material loss of complete biodegradation and poor compatibility, and achieve the effects of prolonging effective control time, reducing particle size and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A preparation method of biodegradable emamectin benzoate microspheres, comprising the following steps:
[0030] (1) Preparation of drug-containing oil phase suspension: mix 0.3000g emamectin benzoate technical drug with 0.3g PLA / PPC (mass ratio 4 / 6), add 7mL dichloromethane, ice-water bath dissolved in medium to obtain a drug-containing oil phase suspension; wherein, the active ingredient in the original drug of emamectin benzoate accounts for 95.8%;
[0031] (2) Preparation of primary emulsion: using a high-speed homogenizer, inject the drug-containing oil phase suspension in step (1) into 100 mL of PVA-1788 aqueous solution under the condition of uniform dispersion of milk, wherein, PVA-1788 The content in the aqueous solution is 10 mg / ml, and the O / W primary emulsion is prepared; the emulsification conditions are: the temperature is 30°C, the dispersion speed is 7000rpm, and the emulsification time is 5min;
[0032] (3) Preparation of microspheres: Stir the primary ...
Embodiment 2
[0035] A preparation method of biodegradable emamectin benzoate microspheres, comprising the following steps:
[0036] (1) Preparation of medicinal oil phase suspension: mix 0.3000g emamectin benzoate technical drug with 0.9000g PLA / PPC (mass ratio 6 / 4, add 10 mL dichloromethane, ice-water bath dissolved in medium to obtain a drug-containing oil phase suspension; wherein, the active ingredient in the original drug of emamectin benzoate accounts for 95.8%;
[0037] (2) Preparation of primary emulsion: use a high-speed homogenizer to inject the drug-containing oil-phase suspension in step (1) into 100 mL of aqueous gum arabic solution under the condition of homogenous emulsion dispersion, wherein the gum arabic gum in the aqueous solution O / W primary emulsion was prepared with the content of 7.5 mg / ml in it; the emulsification conditions were: temperature 30 ℃, dispersion speed 10000 rpm, emulsification time 1.5 min;
[0038] (3) Preparation of microspheres: Pour all the primary ...
Embodiment 3
[0041] A preparation method of biodegradable emamectin benzoate microspheres, comprising the following steps:
[0042] (1) Preparation of drug-containing oil phase suspension: mix 0.6000g emamectin benzoate technical drug with 0.3000g PLA / PPC (mass ratio 8 / 2), add 10mL dichloromethane, and put in an ice-water bath dissolved in medium to obtain a drug-containing oil phase suspension; wherein, the active ingredient in the original drug of emamectin benzoate accounts for 95.8%;
[0043] (2) Preparation of primary emulsion: using a high-speed homogenizer, inject the drug-containing oil-phase suspension in step (1) into 100 mL of PVA-1788 aqueous solution under the condition of homogeneous emulsion dispersion, wherein, PVA-1788 The content in the aqueous solution is 10 mg / ml, and the O / W primary emulsion is prepared; the emulsification conditions are: the temperature is 30°C, the dispersion speed is 8000 rpm, and the emulsification time is 4 minutes;
[0044] (3) Preparation of mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com