Preparation and application of a C5/C6 normal paraffin isomerization catalyst
A technology of n-alkane and catalyst, which is applied in the application field of catalyst in the isomerization reaction of C5/C6 n-alkane, and can solve the problems of catalyst trial and effect being difficult to apply and consider, high equipment requirements, and small molecular weight, etc. Achieving clearly innovative, low-cost, mild-condition results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
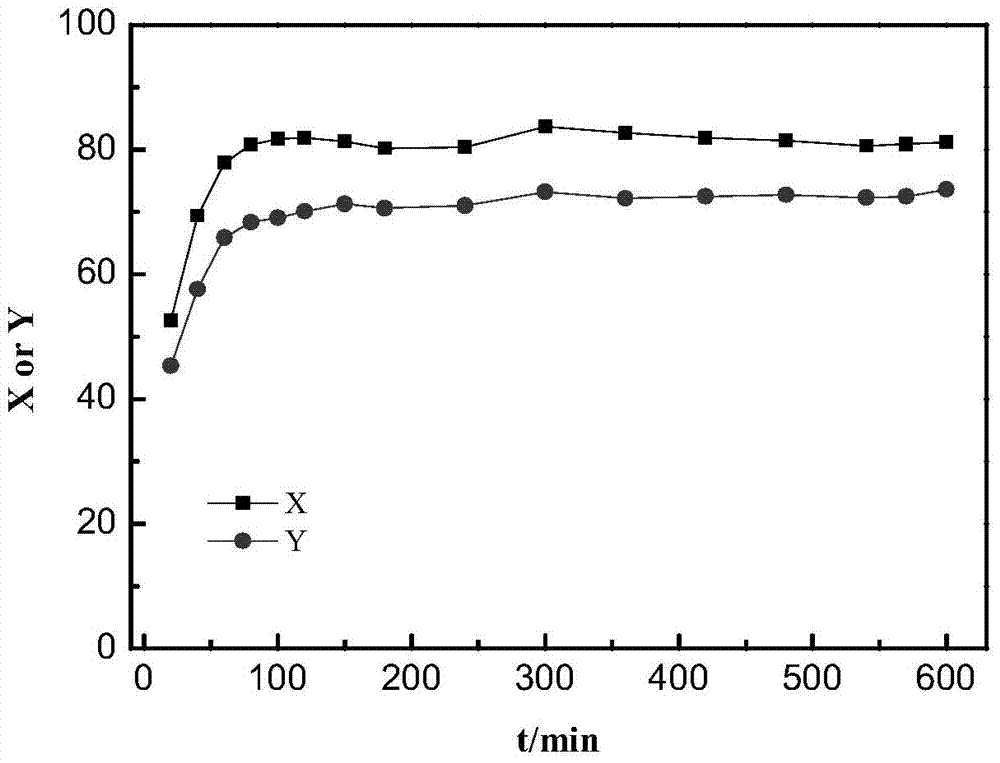
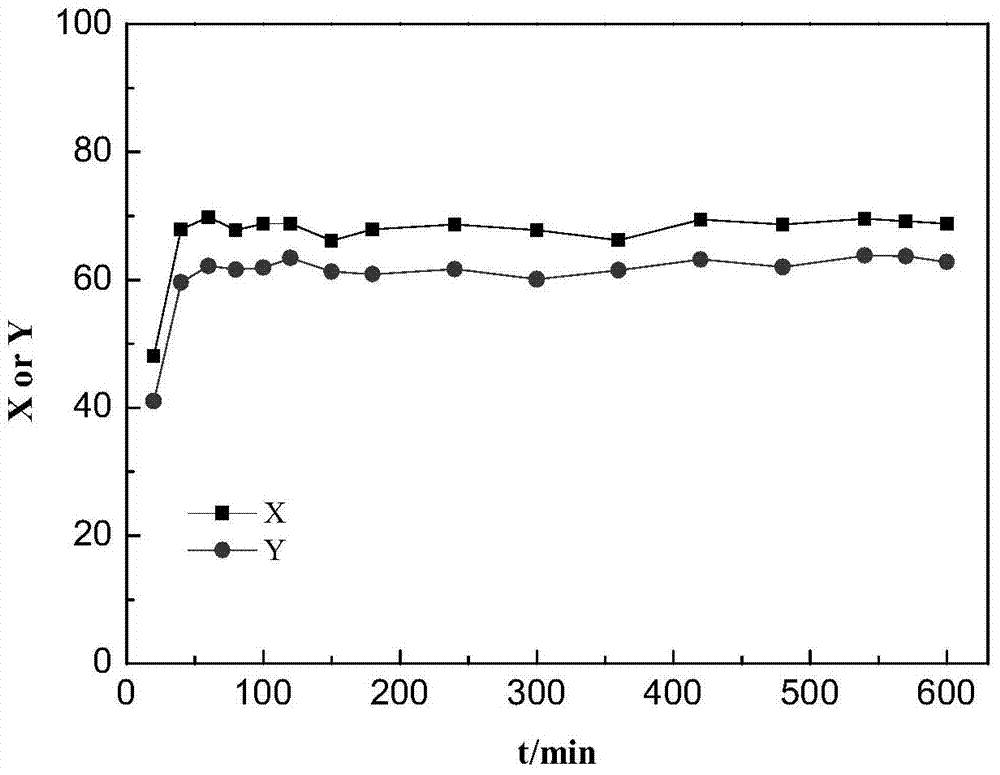
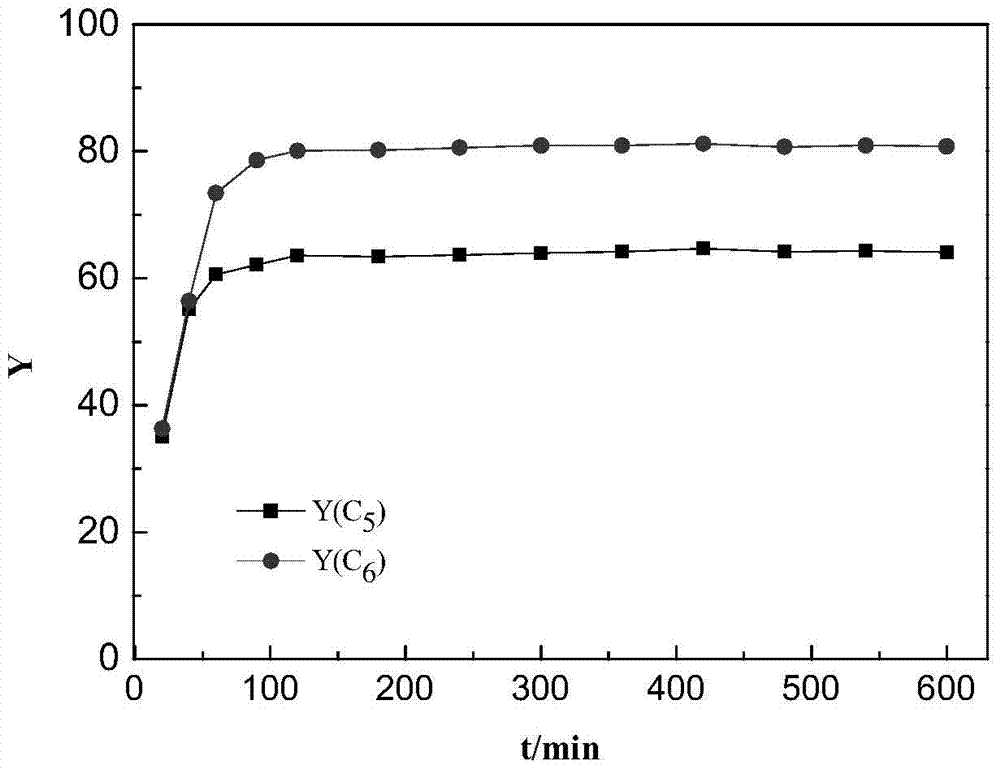
[0034] Embodiment 1. At first 8.4g of sodium hypophosphite monohydrate was added to 50mL of deionized water under stirring at room temperature, and after dissolving, 9.4g of nickel chloride hexahydrate was added (the phosphorus / nickel mol ratio was 2 at this moment). After stirring and dissolving, the obtained clear solution was put into a petri dish and dried at 90°C. Then heat-treat the dried precursor powder at 150° C. to 300° C. for more than half an hour under airtight conditions, and cool to room temperature to obtain a black solid. Wash with water more than 3 times. Pour the black solid into an evaporating dish, put it into an oven, and dry it at 80°C, and then the active phase nickel phosphide is obtained.
[0035] Weigh 12g H-Beta molecular sieve powder, 4gγ-Al 2 o 3 Add the powder into a mortar, grind and mix well. Weigh 0.64g of the above-prepared nickel phosphide, add it evenly in multiple times, and grind evenly. Spray an appropriate amount of acetic acid solut...
Embodiment 2
[0037] The preparation process of embodiment 2. catalyzer is the same as embodiment 1, and difference is that the phosphorus / nickel mol ratio of the sodium hypophosphite and nickel salt mixed solution adopted in the preparation catalyzer process is 5, and active phase phosphating in the prepared catalyzer The mass fraction of nickel is 4%. The implementation conditions of the n-hexane hydroisomerization of the catalyst are the same as in Example 1. As a result of the analysis, the conversion rate of n-hexane was 78.1%, and the isomerization selectivity was 89.7%.
Embodiment 3
[0038] The preparation process of embodiment 3. catalyzer is the same as embodiment 1, and difference is that the phosphorus / nickel mol ratio of the sodium hypophosphite and nickel salt mixed solution adopted in the preparation catalyzer process is 1, and active phase phosphating in the prepared catalyzer The mass fraction of nickel is 4%. The implementation conditions of the n-hexane hydroisomerization of the catalyst are the same as in Example 1. As a result of the analysis, the conversion rate of n-hexane was 81.3%, and the isomerization selectivity was 85.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com