A kind of formononetin derivative, preparation method and use thereof
The technology of a compound and structural formula is applied in the field of formononetin derivatives and their preparation, which can solve the problems of limited curative effect and large side effects, and achieve the effects of reducing content, preventing and treating pulmonary fibrosis, and increasing total antioxidant capacity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
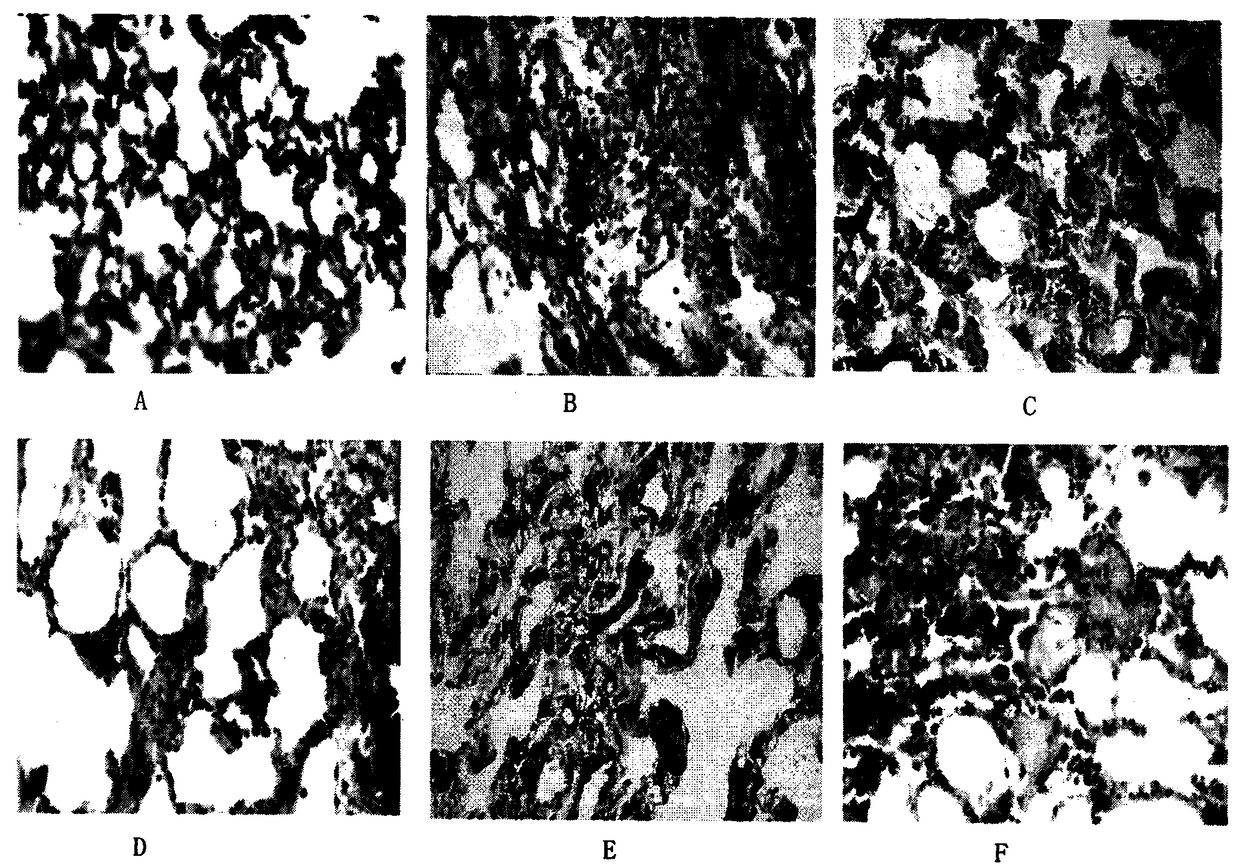
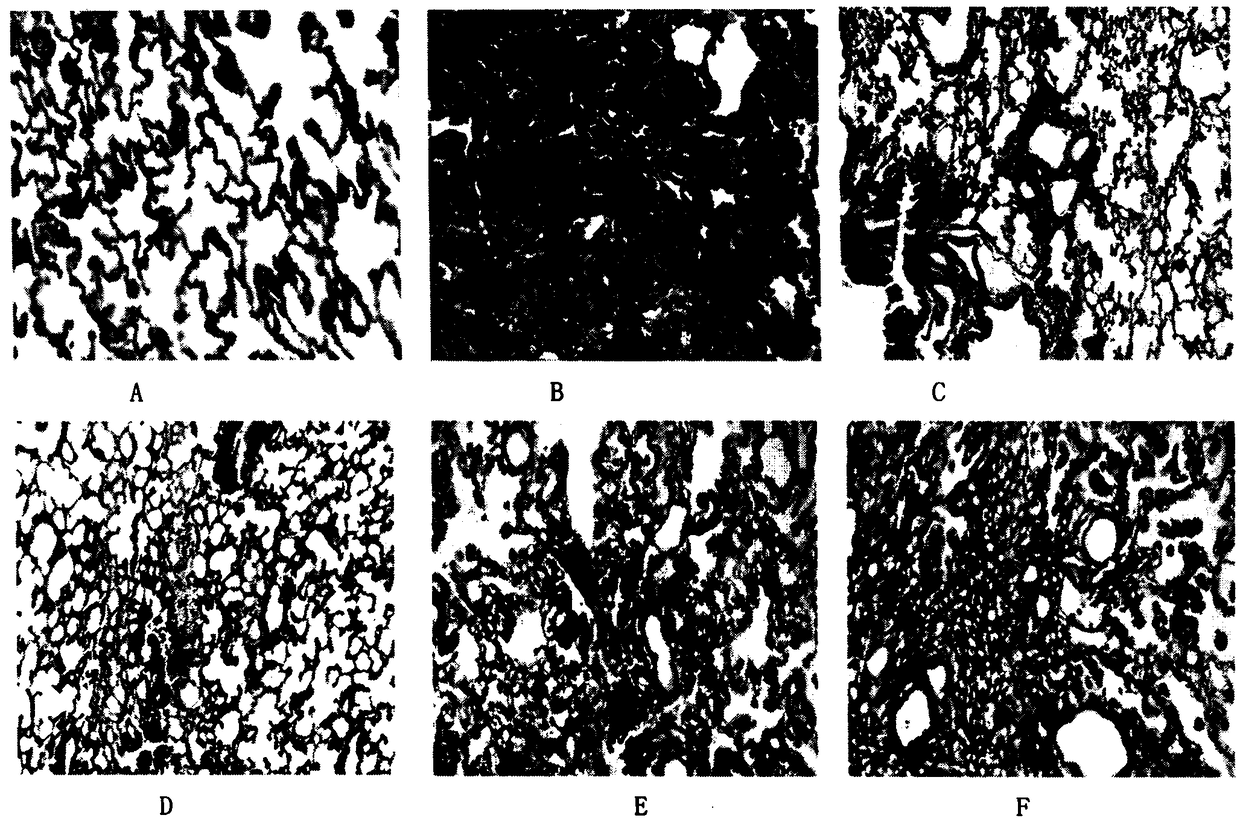
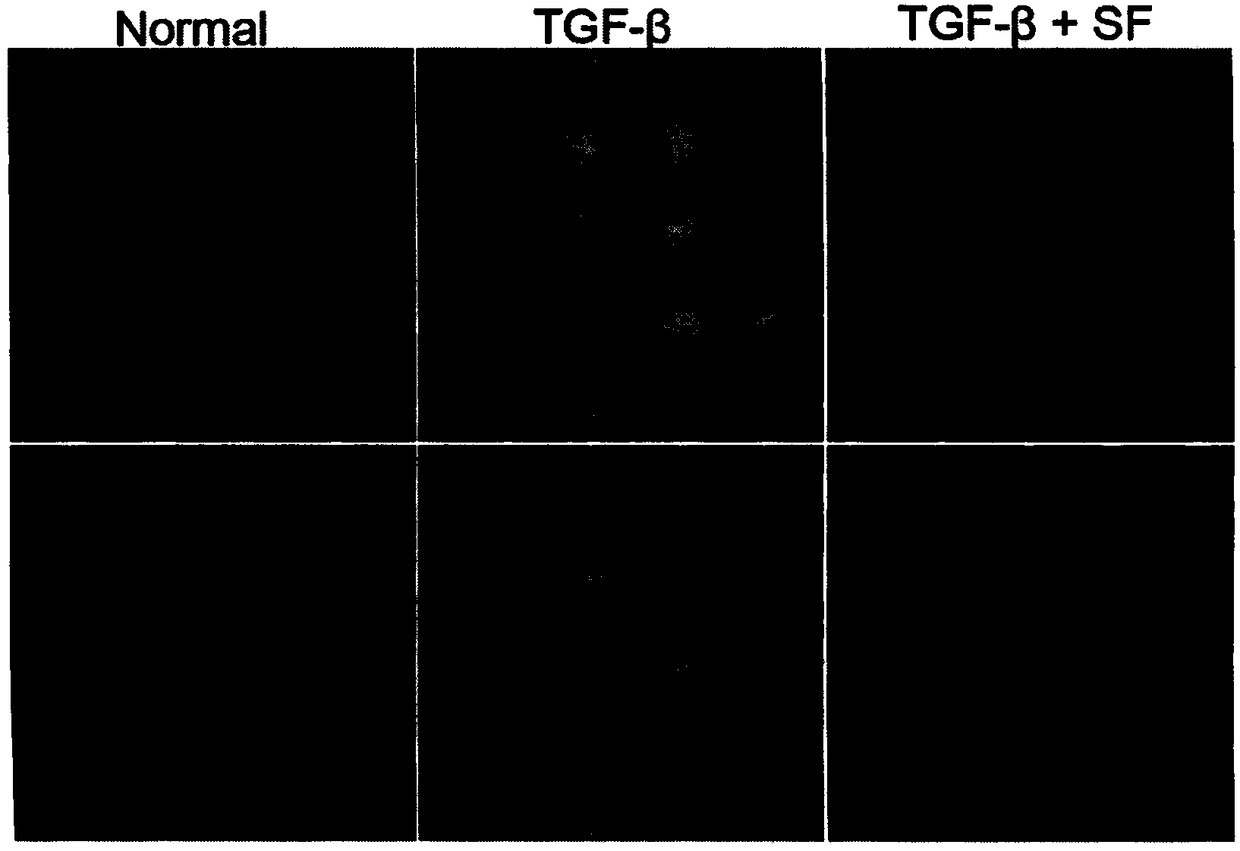
Image
Examples
Embodiment 1
[0019] Embodiment 1. Preparation of formononetin-7-salvianolic acid ester
[0020] Add formononetin to 4 times the amount of pyridine, stir to dissolve, add 1.2 times the amount of danshensu, and add DCC as an activator at a ratio of 1%, and react for 1 hour. The reaction solution was poured into 30 times the amount of saturated NaCl solution, allowed to stand, precipitated, and suction filtered. After the filter cake is washed with 20 times the amount of saturated NaCl solution to be nearly neutral, add 10 times the amount of water, heat and stir to dissolve, filter while it is hot, refrigerate at low temperature for crystallization, and filter. Add 10 times the amount of water to the filter cake, heat to dissolve, filter while it is hot, refrigerate at low temperature to crystallize, and filter. The filter cake was recrystallized with 8 times the amount of 70% ethanol, filtered, dried under reduced pressure at 100°C, and pulverized to obtain the obtained product.
[0021] ...
Embodiment 2
[0022] Example 2. Preparation of formononetin-7-salvianolic acid ester
[0023] Add formononetin to 10 times the amount of acetone, stir to dissolve, add 2.0 times the amount of danshensu, and add DMAP as an activator at a ratio of 5%, and react for 1 hour. The reaction solution was poured into 30 times the amount of saturated NaCl solution, allowed to stand, precipitated, and suction filtered. After the filter cake is washed with 20 times the amount of saturated NaCl solution to be nearly neutral, add 20 times the amount of water, heat and stir to dissolve, filter while it is hot, refrigerate at low temperature for crystallization, and filter. Add 20 times the amount of water to the filter cake, heat to dissolve, filter while it is hot, refrigerate at low temperature to crystallize, and filter. The filter cake was recrystallized with 10 times the amount of 95% methanol, filtered, dried under reduced pressure at 60°C, and pulverized to obtain the obtained product.
[0024] I...
Embodiment 3
[0025] Example 3. Preparation of formononetin-7-salvianolic acid ester
[0026] Add formononetin into 10 times the amount of chloroform, stir to dissolve, add 1.0 times the amount of danshensu, and add 1% EDCI as an activator, and react for 3 hours. The reaction solution was poured into 30 times the amount of saturated NaCl solution, allowed to stand, precipitated, and suction filtered. After the filter cake is washed with 20 times the amount of saturated NaCl solution to be nearly neutral, add 20 times the amount of water, heat and stir to dissolve, filter while it is hot, refrigerate at low temperature for crystallization, and filter. Add 20 times the amount of water to the filter cake, heat to dissolve, filter while it is hot, refrigerate at low temperature to crystallize, and filter. The filter cake was recrystallized with 8 times the amount of 95% acetone, filtered, dried under reduced pressure at 60°C, and pulverized to obtain the obtained product.
[0027] In order to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com