Application of fused immune protein to preparation of medicine for treating multiple sclerosis
A multiple sclerosis and immunoglobulin technology, applied in the field of bioengineering, can solve problems such as toxic side effects, large differences in patient tolerance, and rebound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
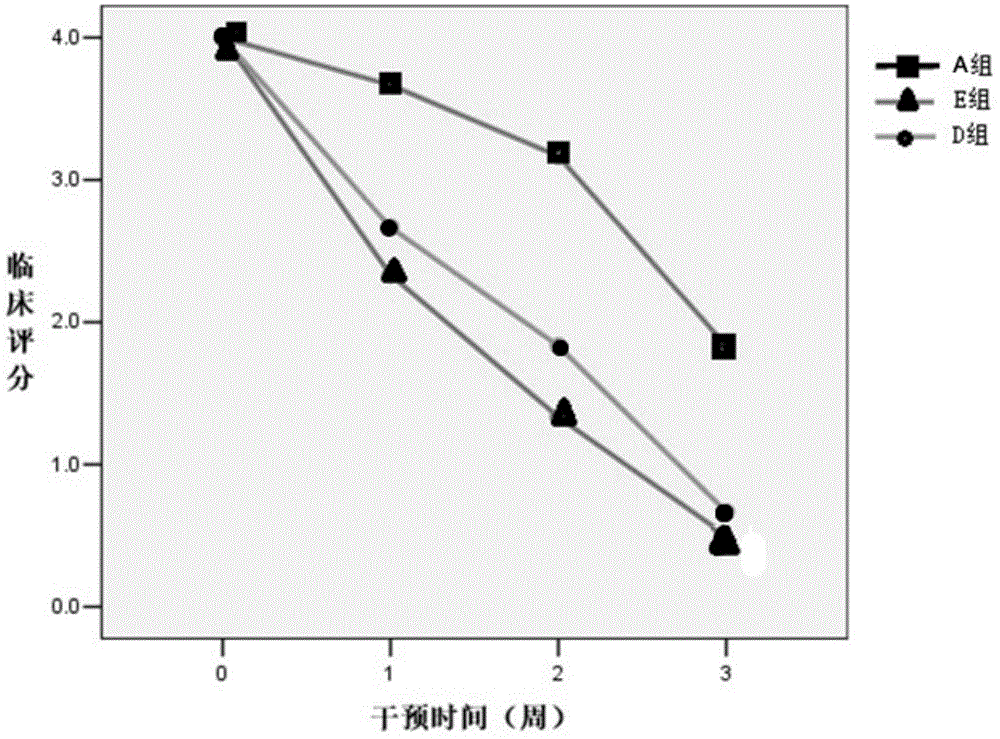
[0049] Example 1: The incidence of experimental mice and the average clinical score at different stages
[0050] When the experiment was terminated on the 42nd day, all the mice in group A had no disease; except for the 24 mice that were sacrificed before the onset of the C57BL / 6J model mice in group C, 41 / 48 (85.4%) of the mice developed the disease. Group C developed symptoms successively 15-20 days after inoculation, peaked at 24-28 days, and maintained for 5-7 days. Some clinical symptoms of the natural course group could be alleviated, and the clinical scores were relatively lower, but the symptoms persisted, and then entered chronic maintenance. In the second stage, the disease showed a typical chronic non-remission process; 29 / 36 (80.5%) mice in group D had the disease, and 28 / 36 (77.8%) mice in group E had the disease; the symptoms were the same as those in the EAE group. One mouse in group E died without disease, which may be related to improper operation during injec...
Embodiment 2
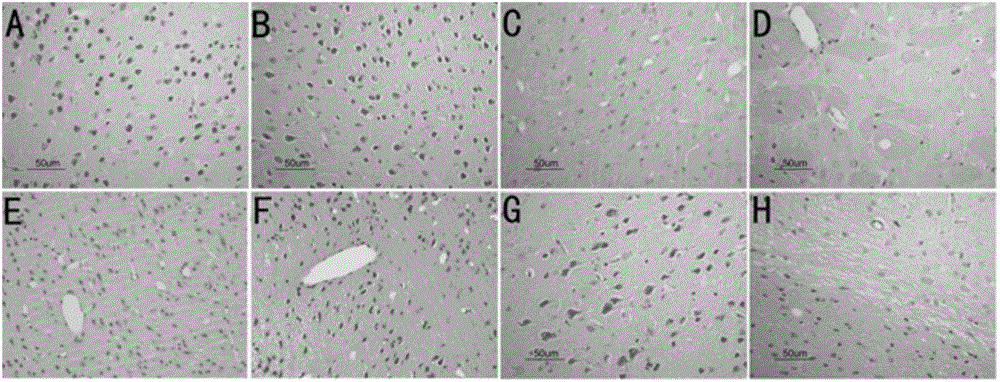
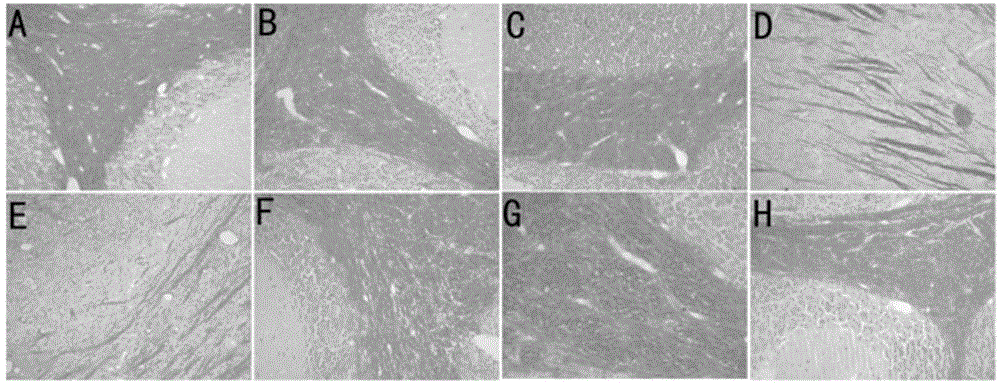
[0055] Example 2: Histopathological and immunohistochemical changes in experimental mouse brain
[0056] After treatment, mouse brain HE and LFB staining can be seen under light microscope (see figure 2 and image 3 ). The infiltration of inflammatory cells was better than that in the peak period and chronic maintenance period of the EAE group, and the demyelination was also alleviated. Abnormal staining due to local aggregation of APP due to axonal damage was not seen. The degree of activation of microglial cells in the brain was significantly reduced in the two treatment groups, and almost returned to normal after treatment with methylprednisolone; it shows that methylprednisolone and fusion protein have anti-inflammatory and anti-myelin damage effects, especially the effect of methylprednisolone more obvious.
[0057] figure 2 A in A is the HE staining of the brains of the mice in the control group; B is the HE staining of the brains of the mice in the EAE group in t...
Embodiment 3
[0065] Example 3: Changes in the permeability of the blood-brain barrier
[0066] Such as Figure 9 As shown in Table 2, the EAE group has always maintained a high level at the peak of the onset and the chronic maintenance period, and no significant changes have been observed after fusion immunoglobulin treatment, and there is no statistical difference compared with the peak of the onset and the chronic maintenance period (P>0.05 ), the permeability of the blood-brain barrier in the methylprednisolone group decreased, which was statistically different from that in the EAE group (P<0.05; indicating that methylprednisolone can inhibit the increase in the permeability of the blood-brain barrier, while fusion immune Globulin did not show this effect.
[0067] Table 2 Changes of EB content (μg / g brain tissue) in hippocampal tissue of experimental mice
[0068]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com