Preparation method of flame retardant trihydroxymethylphosphine oxide caged phosphite compound
A technology of trimethylol and phosphite, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the safety hazards of toxic gases and smoke, and halogen flame retardants Problems such as limited application range, to achieve the effect of good symmetry of the cage ring structure, easy large-scale transformation and production, and good flame retardancy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
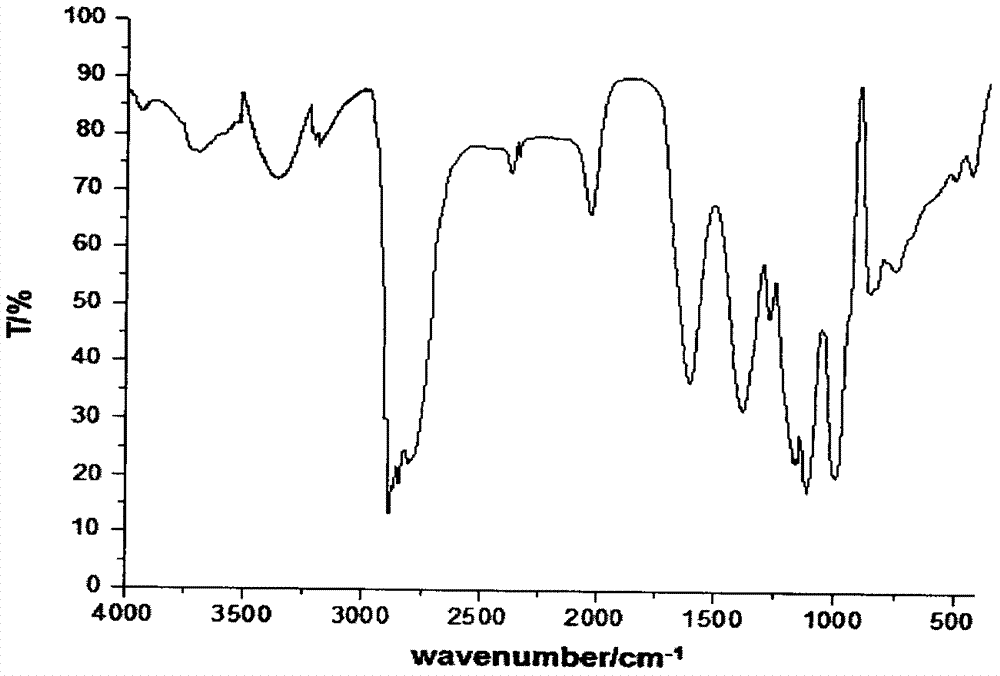
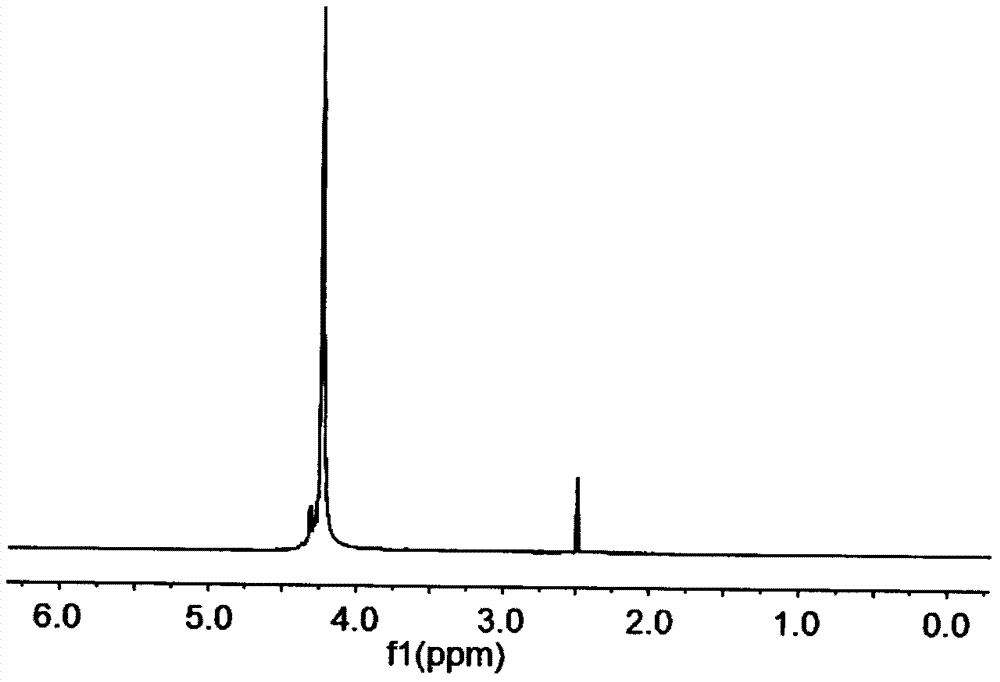
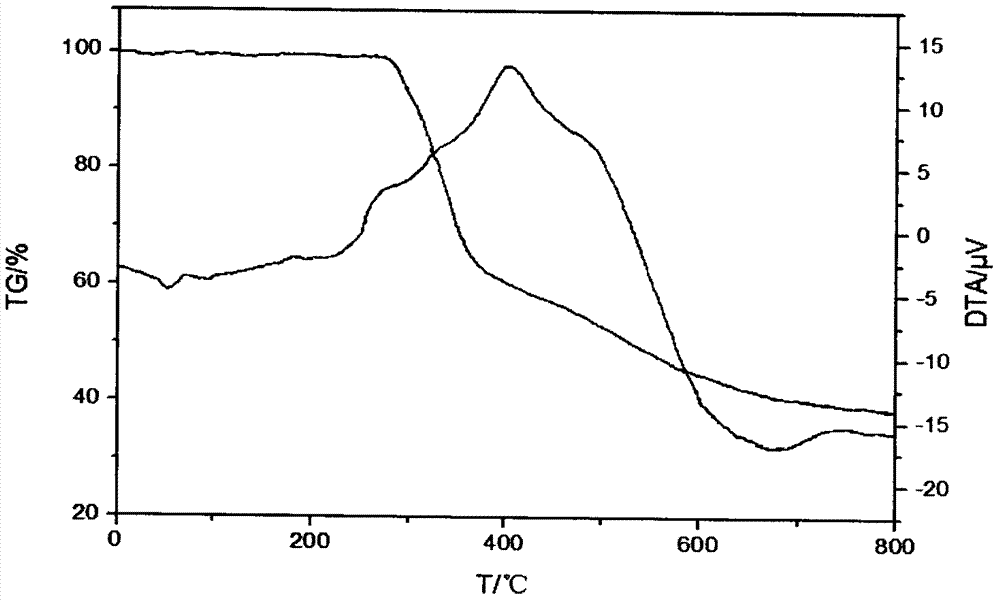
Image
Examples
Embodiment 1
[0025] Example 1 In a 100ml four-neck flask equipped with a stirrer, a thermometer, a high-efficiency reflux condenser and a hydrogen chloride absorption device connected to the upper mouth of the condenser, the air in the bottle was driven away with nitrogen, and 30ml of acetonitrile, 14.00g (0.1 mol) trihydroxymethyl phosphine oxide, cooled to below 0°C, add 13.73g (0.1mol) phosphorus trichloride dropwise under stirring, make it fully mixed, heat up to 10°C, hydrogen chloride begins to be released, heat up to 25°C Keep warm for 1 hour, then raise the temperature to 80°C and keep warm for 3 hours. After no hydrogen chloride is released, remove acetonitrile by distillation under reduced pressure (for recycling), then add 50ml of ice water, and add 5% sodium carbonate solution dropwise under stirring to make the pH of the reaction system = 7. The product solid was dispersed in water, filtered, the filter cake was rinsed with 10 ml of ice water, compacted and drained, and the fil...
Embodiment 2
[0026] Example 2 In a 100ml four-necked flask equipped with a stirrer, a thermometer, a high-efficiency reflux condenser and a hydrogen chloride absorption device connected to the upper mouth of the condenser, use nitrogen to exhaust the air in the bottle, add 40ml of benzene, 14.00g (0.1 mol) trihydroxymethyl phosphine oxide, cooled to below 0°C, add 13.73g (0.1mol) phosphorus trichloride dropwise under stirring, make it fully mixed, heat up to 10°C, hydrogen chloride begins to be released, heat up to 25°C Keep warm for 1 hour, then heat up to 75°C and keep warm for 4 hours. After no hydrogen chloride is released, benzene is removed by distillation under reduced pressure (recycled), then add 45ml of ice water, and add 5% sodium carbonate solution dropwise under stirring to make the reaction system pH= 7. The product solid was dispersed in water, filtered, the filter cake was rinsed with 10 ml of ice water, compacted and drained, and the filter cake was vacuum-dried to obtain a...
Embodiment 3
[0027] Example 3 In a 150ml four-neck flask equipped with a stirrer, a thermometer, a high-efficiency reflux condenser and a hydrogen chloride absorption device connected to the upper mouth of the condenser, the air in the bottle was driven away with nitrogen, and 50ml of tetrahydrofuran was added, 14.00g (0.1 mol) trihydroxymethyl phosphine oxide, cooled to below 0°C, add 13.73g (0.1mol) phosphorus trichloride dropwise under stirring, make it fully mixed, heat up to 10°C, hydrogen chloride begins to be released, heat up to 25°C Keep warm for 1 hour, then raise the temperature to 65°C and keep it warm for 5 hours. After no hydrogen chloride is released, distill under reduced pressure to remove tetrahydrofuran (recycled), then add 40ml of ice water, add 5% sodium carbonate solution dropwise under stirring, and make the pH of the reaction system = 7. The product solid was dispersed in water, filtered, the filter cake was rinsed with 10 ml of ice water, compacted and drained, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| limiting oxygen index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com