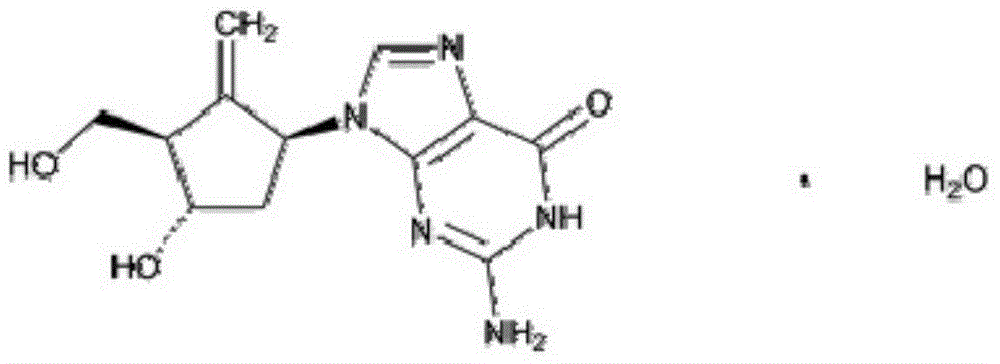
Entecavir and mannatide drug composition and preparation method thereof
A technology of mannan peptide and entecavir, which is applied in the field of medicine to achieve good synergistic effect and pharmacological activity, and enhance immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
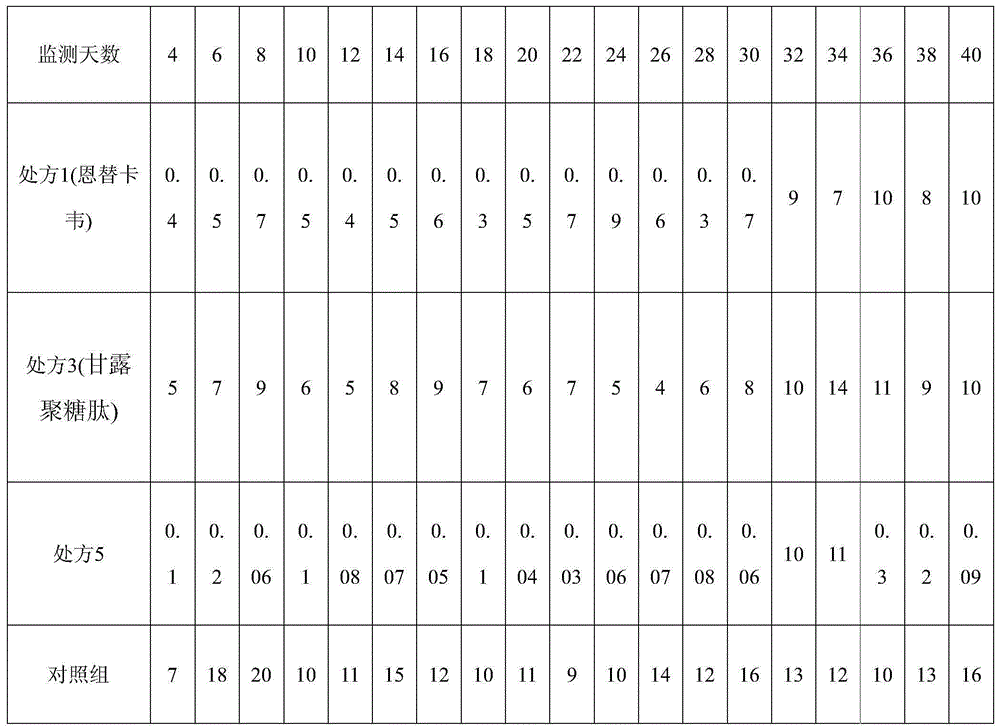
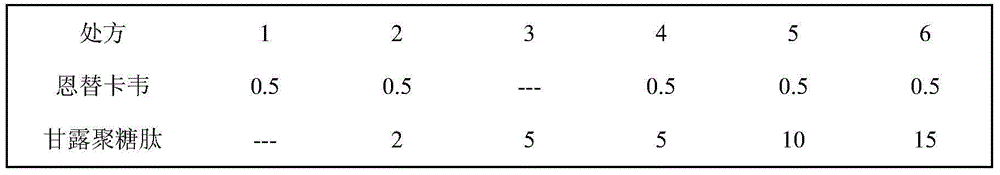
[0036] Embodiment 1 Entecavir and mannan peptide pharmaceutical composition formulation prescription ratio
[0037] Table 1: The formulation ratio of entecavir and mannan peptide pharmaceutical composition for the treatment of viral hepatitis B, which can be used in both tablets and hard capsules.
[0038] The general manufacturing method for solid dosage forms is as follows:
[0039] 1. The proportioned entecavir, mannan peptide and excipients are mixed together;
[0040] 2. The homogeneous matter obtained in step 1 is further compacted into small particles;
[0041] 3. Lubricants such as magnesium stearate etc. are mixed with the granules obtained in the above step 2;
[0042] 4. The homogenate in step 3 is compressed into tablets or other solid dosage forms;
[0043] 5. If necessary, the coating liquid can be sprayed onto the tablet obtained in step 4 in the form of mist droplets.
[0044] When adopting hard capsule preparation, then the homogenate obtained in step 3 is d...
Embodiment 2
[0047] Prescription and preparation of embodiment 2 pharmaceutical composition tablet
[0048] prescription
[0049]
[0050] Preparation method: Weigh each component according to the prescription amount, mix thoroughly and evenly, granulate, and compress into tablets with a tablet machine.
Embodiment 3
[0051] Prescription and preparation of embodiment 3 pharmaceutical composition capsules
[0052] prescription
[0053]
[0054] Preparation method: weigh each component according to the prescription amount, sieve and dry, fully mix, granulate, and fill with hollow capsules to obtain capsules of the pharmaceutical composition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com