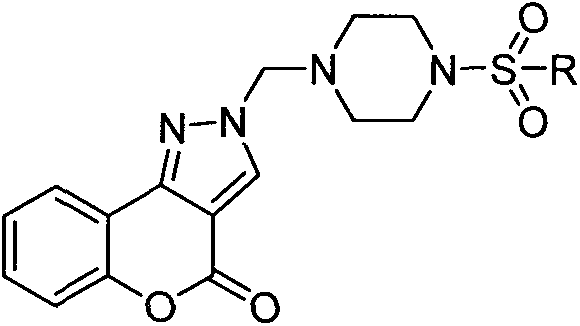
Sulfonyl piperazine containing coumarin pyrazole compounds, preparation of coumarin pyrazole compounds and application of coumarin pyrazole compounds in tumor cell inhibition
A technology containing sulfonylpiperazine and coumarin, which can be used in antineoplastic drugs, drug combinations, organic chemistry, etc., and can solve problems such as cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Preparation of 2-((4-(benzenesulfonyl)piperazin-1-yl)methyl)chromeno[4,3-c]pyrazol-4(2H)-one (Compound 1)
[0018]
[0019] 0.06 mmol of 4-hydroxycoumarin was dissolved in 46.2 ml of anhydrous N,N-dimethylformamide, and the reaction liquid was cooled to below 0°C. Under the condition below 0°C, slowly add POCl dropwise while stirring 3 0.18mmol, to POCl 3 After the addition was complete, the reaction solution was slowly warmed up to room temperature and stirred for 0.5 h, then heated up to 65-70° C. and stirred for 6 h. The reaction was tracked and monitored by TLC. After the reaction was completed, the reaction solution was poured into 200 g of ice-water mixture and stirred vigorously, and a large amount of light yellow solid 1 was precipitated. The solid was suction filtered and washed with 5% Na 2 CO 3 The aqueous solution was washed, and the obtained solid was dried to obtain the intermediate.
[0020] Dissolve 1 mmol of the solid obtained in step...
Embodiment 2
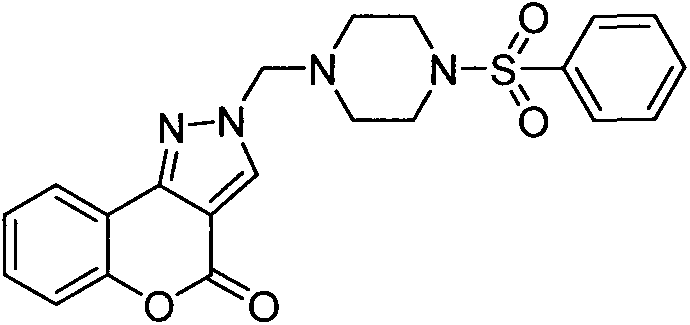
[0023] Example 2: Preparation of 2-((4-tosylpiperazin-1-yl)methyl)chromeno[4,3-c]pyrazol-4(2H)-one (Compound 2)
[0024]
[0025] The preparation method is the same as in Example 1. The product is a white powdery solid. Yield 57%. Mp: 176-177°C. 1 HNMR (400MHz, CDCl 3 ): 8.20(s, 1H, ArH), 8.05(d, J=7.72Hz, 1H, ArH), 7.61(d, J=7.92Hz, 2H, ArH), 7.49(t, J=7.70Hz, 1H, ArH), 7.38(d, J=8.28Hz, 1H, ArH), 7.31(t, J=9.66Hz, 3H, ArH), 5.04(s, 2H, -CH 2 ), 3.05(s, 4H, -CH 2 CH 2 ), 2.75(t, J=4.38Hz, 4H, -CH 2 CH 2 ), 2.40 (s, 3H, CH 3 ).MS (ESI): 439.32 (C 22 h 23 N 4 o 4 S, [M+H] + ).Anal.CalcdforC 22 h 22 N 4 o 4S: C, 60.26; H, 5.06; N, 12.78%. Found: C, 60.27; H, 5.08; N, 12.76%.
Embodiment 3
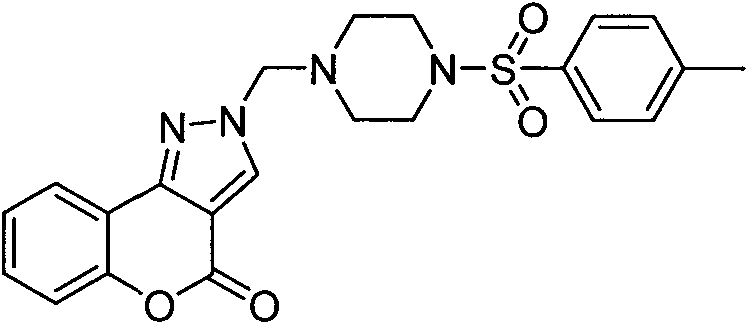
[0026] Example 3: 2-((4-((4-methoxyphenyl)sulfonyl)piperazin-1-yl)methyl)chromeno[4,3-c]pyrazole-4(2H) - Preparation of ketone (compound 3)
[0027]
[0028] The preparation method is the same as in Example 1. The product is a white powdery solid. Yield 54%. Mp: 182-184°C. 1 HNMR (400MHz, CDCl 3 ): 8.20(s, 1H, ArH), 8.05(d, J=7.64Hz, 1H, ArH), 7.66(d, J=8.36Hz, 2H, ArH), 7.48(t, J=5.76Hz, 1H, ArH), 7.38(d, J=8.24Hz, 1H, ArH), 7.32(t, J=7.52Hz, 1H, ArH), 6.96(d, J=8.52Hz, 2H, ArH), 5.05(s, 2H ,-CH 2 ), 3.85(s, 3H, OCH 3 ), 3.05(s, 4H, -CH 2 CH 2 ), 2.74 (d, J=4.32Hz, 4H, -CH 2 CH2). MS (ESI): 455.37 (C 22 h 23 N 4 o 5 S, [M+H] + ).Anal.CalcdforC 22 h 22 N 4 o 5 S: C, 58.14; H, 4.88; N, 12.33%. Found: C, 58.15; H, 4.91; N, 4.86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com