Application of Pedinophyllol B in preparation of vascular protective drug
A technology of pedinophyllolb, 1. pedinophyllolb is applied in the application field of Pedinophyllol B in the preparation of vascular protective drugs, and can solve problems such as no reports of compound activity yet.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
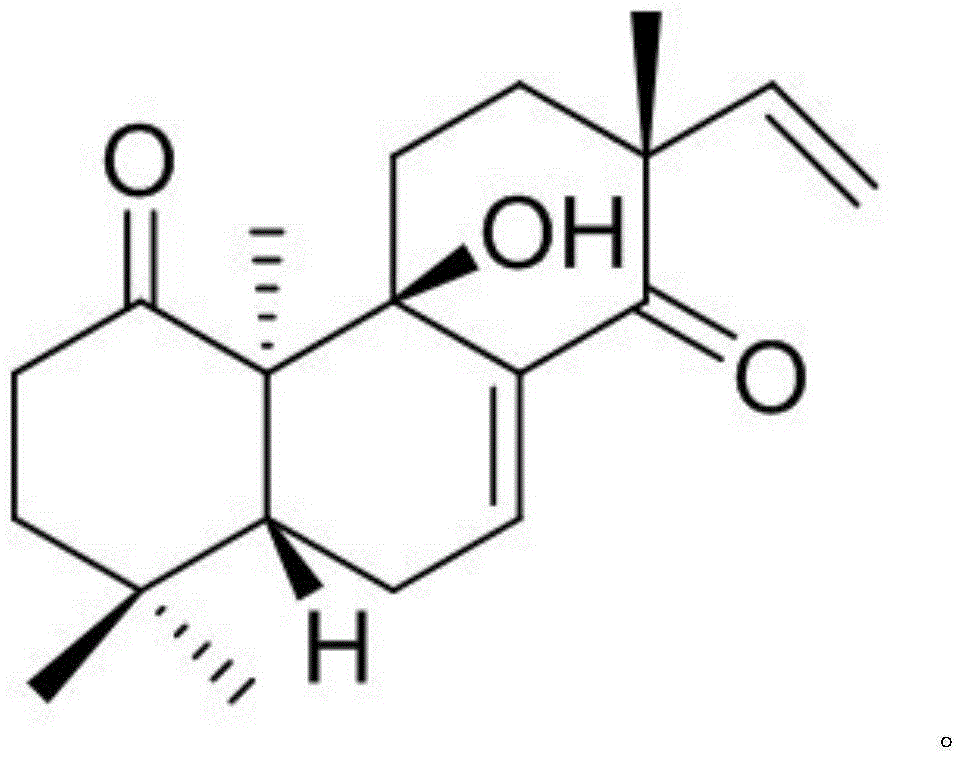
[0009] Example 1: Isolation, Preparation and Structure Confirmation of PedinophyllolB
[0010] The preparation method of PedinophyllolB is the same as that reported in the literature (HighlyOxygenatedent-Pimarane-TypeDiterpenoids from the Chinese Liverwort Pedinophyllum interruptum and Their Allelopathic Activities, J.Nat.Prod., 2013, 76, 1647-1653).
[0011] Structural confirmation: the molecular formula is C 20 h 28 o 3 , with an unsaturation of 7. H NMR spectrum data δ H (ppm, DMSO-d 6 , 500MHz): H-2 (2.82, dt, J=4.9, 13.3), H-2 (2.23, m), H-3 (1.79-1.83, m), H-5 (2.39, dd, J=3.8 , 12.6), H-6 (2.28, m), H-7 (6.80, d, J=4.8), H-11 (1.96, dt, J=17.9, 2.5), H-11 (2.36, m), H-12 (1.73, m), H-12 (2.24, m), H-15 (5.70, dd, J=10.7, 17.6), H-16 (5.07, d, J=10.7), H-16 ( 4.83, d, J=17.6), H-17 (1.23, s), H-18 (1.02, s), H-19 (1.19, s), H-20 (1.19, s); C NMR data δ C (ppm, DMSO-d 6 , 150Hz): 218.5 (C, 1-C), 37.1 (CH 2 , 2-C), 41.5 (CH 2 , 3-C), 32.7(C, 4-C), 44.2(CH, 5-C)...
Embodiment 2
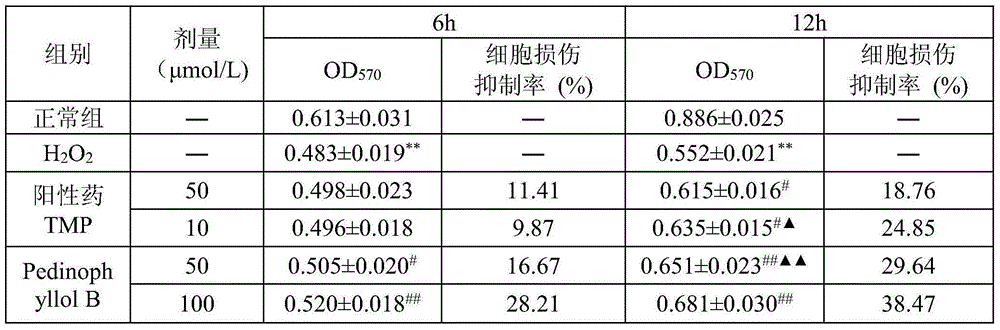
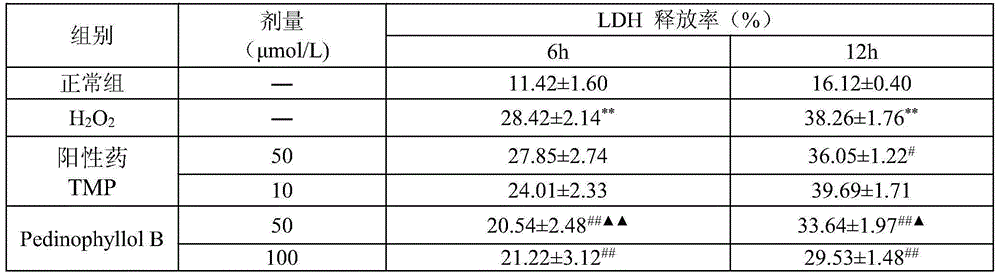
[0012] Embodiment 2: Pharmacological action test of Pedinophyllol B
[0013] 1. Materials and Instruments
[0014] Vascular endothelial cells (ECV-304) were donated by the Department of Immunology, School of Medicine, Shandong University. PedinophyllolB is self-made, and the HPLC normalized purity is greater than 98%. RPMI-1640 dry powder medium and trypsin were purchased from Gibeo, USA. Fetal bovine serum was purchased from Tianjin TBD Company. MTT, agarose, and AilllexinV-FITC were purchased from Sigma, USA. LDH, MDA, SOD, GSH-Px assay kits were purchased from Nanjing Jiancheng Bioengineering Institute. DMSO was purchased from China Pharmaceutical (Group) Shanghai Chemical Reagent Co., Ltd. Hematoxylin was purchased from Fuzhou Maixin Biotechnology Co., Ltd. Rhodamine123 was purchased from Solarbic Corporation of the United States. The positive drug ligustrazine was purchased from Shanghai Yuanye Biotechnology Co., Ltd.
[0015] Inverted optical microscope (Japan Ol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com