Application of aphanalide J to preparation of drugs for treating liver cancer
A technology for the treatment of liver cancer and drugs, which is applied in the application field of Aphanalide J in the preparation of drugs for the treatment of liver cancer, and can solve the problems that there are no reports on the activity of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
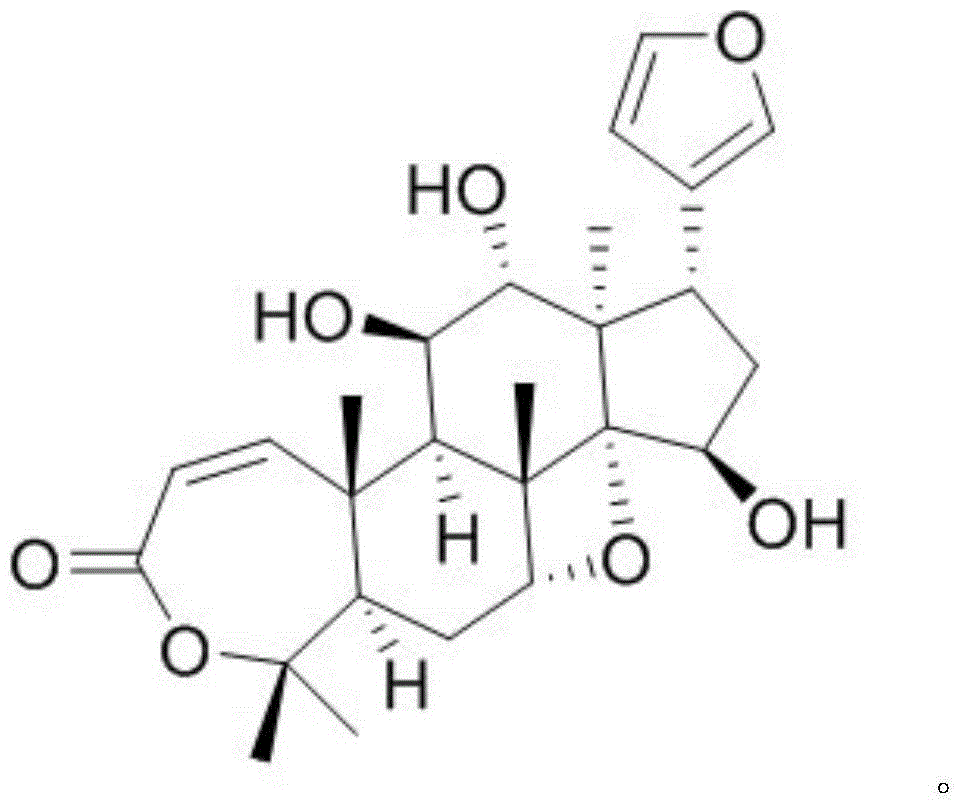
[0011] Example 1: Isolation, preparation and structure confirmation of Aphanalide J
[0012] The preparation method of Aphanalide J is the same as that reported in the literature (Bioactive Terpenoids from the Fruits of Aphanamixis grandifolia, J. Nat. Prod., 2013, 76, 1191-1195).
[0013] Confirmation of structure: white amorphous powder, molecular formula is C 26 h 34 o 7 , with an unsaturation of 10. H NMR spectrum data δ H (ppm, DMSO-d 6 , 500MHz): H-1 (6.72, d, J=12.5), H-2 (5.85, d, J=12.5), H-5 (2.29, dd, J=13.2, 4.0), H-6 (1.85 , dd, J=16.0, 4.0), H-6 (1.64, ddd, J=16.0, 13.2, 4.0), H-7 (4.61, s), H-9 (2.60, d, J=4.0), H -11 (3.80, q, J=4.0), H-12 (3.50, t, J=6.5), H-15 (4.51, q, J=3.5), H-16 (1.61, ddd, J=12.0, 10.5, 3.5), H-16 (1.71, dt, J=12.0, 5.5), H-17 (3.25, dd, J=10.5, 5.5), H-18 (0.55, s), H-19 (1.30, s), H-21 (7.30, s), H-22 (6.40, s), H-23 (7.45, s), H-28 (1.23, s), H-29 (1.35, s), H-30 (1.48, s), 11-OH (5.00, d, J=4.0), 12-OH (4.91, d, J=6.5), 15-O...
Embodiment 2
[0014] Embodiment 2: Pharmacological action test of Aphanalide J
[0015] 1. Materials and Instruments
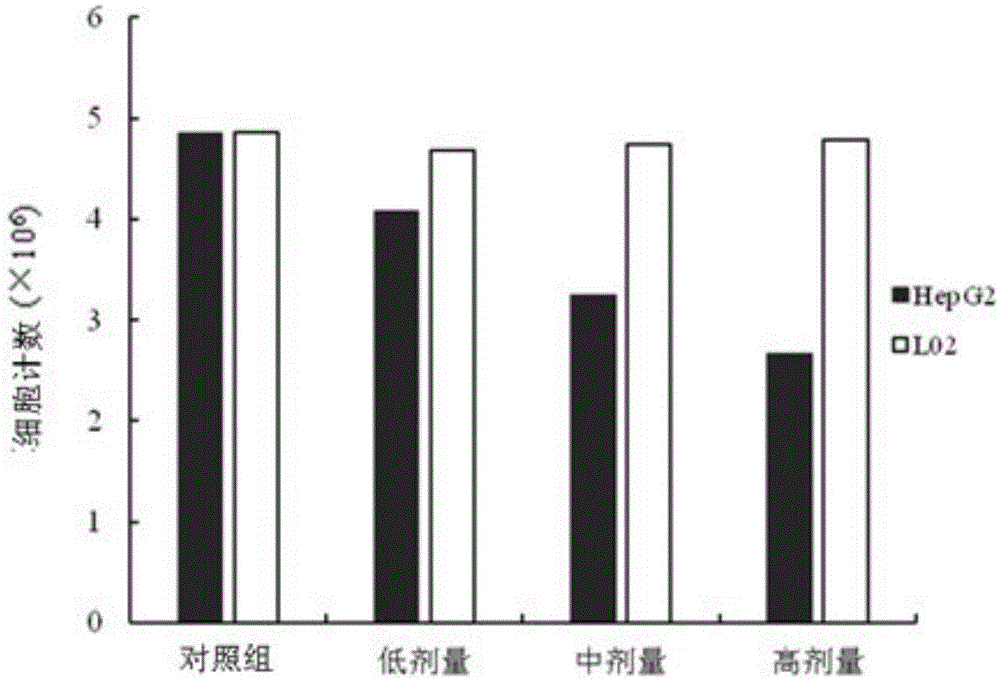
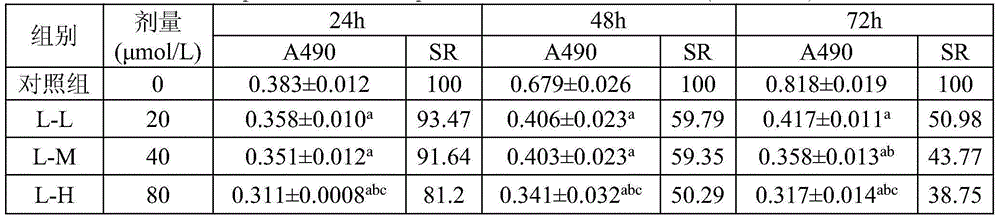
[0016] HepG2 human liver cancer cell line was purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. The L02 normal human liver cell line was donated by the Liver Cancer Institute, Zhongshan Hospital, Fudan University. AphanalideJ is self-made, and the HPLC normalized purity is greater than 98%. RPMI-1640 medium and trypsin were purchased from Gibco. Standard calf serum was purchased from Biowest. 3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide (MTT), dimethylsulfoxide (DMSO), trypan blue (TrypanBLue), dichlorofluorescence Diacetate diacetate (DCFH-DA) was purchased from Shanghai Sinopharm Chemical Reagent Co., Ltd. Other commonly used reagents were of analytical grade.
[0017] Microbalance (Mettler-Toledo, Switzerland, METTLRERAE2000). Ordinary desktop centrifuge (Shanghai Anting Scientific Instrument Factory). Digit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com