Tetrakisglycoluril derivative and preparation method thereof
A technology of tetramethoxymethyl glycoluril and ethoxymethyl glycoluril, which is applied in the fields of coating, organic chemistry, powder coating, etc., can solve the problems of simple spatial structure and poor stability of polymer molecules, and achieve easy-to-obtain raw materials, Conditions are easy to control and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 3g of tetramethoxymethyl glycoluril, 8g of Amberlyst35 resin, and 60g of PGME into a 250ml three-necked flask, raise the temperature to 60°C, stir and react at -0.08MPa for 9 hours, and distill off the generated methanol at the same time.
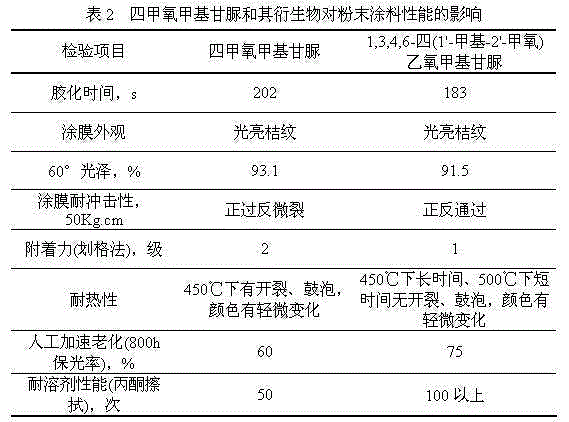
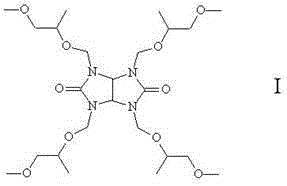
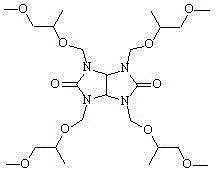
[0020] The reactant was suction-filtered with a Buchner funnel, and the filter residue was washed twice with PGME to recover the resin. The filtrate and washing liquid were combined, and the solvent PGME was recovered by distillation under reduced pressure to obtain 5.12 g of 1,3,4,6-tetrakis(1'-methyl-2'-methoxy)ethoxymethyl glycoluril, with a yield of 98.6% .
[0021] Measured product ESI-MS (m / z): 551.2 (M+1).
[0022] IR(KBr)v / cm -1 : 2929, 1712, 1454, 1392, 1110, 1031, 792.
[0023] 1 H-NMR (CDCl 3 ): δ1.02(d, J=6.3Hz, 12H), δ3.112~3.137(q, 4H), δ3.201(d, J=2.23Hz, 8H), δ3.252(s, 12H), δ3.713~3.748 (m, 8H), δ4.548 (s, 2H).
[0024] 13 C-NMR (CDC1 3 ): δ17.1, δ17.1, δ17.1, δ17.1, δ59.6, δ59.6, δ59.6, δ59.6, δ71.2, δ7...
Embodiment 2
[0026] The Amberlyst15 resin was dried in an oven to 60% of its original weight.
[0027] Add 3g of tetramethoxymethyl glycoluril, 6g of dried Amberlyst15 resin, and 70g of PGME into a 250ml three-necked flask, heat up to 60°C, stir and react at -0.07MPa pressure for 17 hours, and distill off the generated methanol at the same time.
[0028] The reactant was suction-filtered with a Buchner funnel, and the filter residue was washed twice with PGME to recover the resin. The filtrate and washing liquid were combined, and the solvent PGME was recovered by distillation under reduced pressure to obtain 5.19 g of 1,3,4,6-tetrakis(1'-methyl-2'-methoxy)ethoxymethyl glycoluril with a yield of 99.8% .
[0029] Measured product ESI-MS (m / z): 551.0 (M+1).
[0030] IR(KBr)v / cm -1 : 2943, 1712, 1485, 1384, 1024, 995, 790.
[0031] 1 H-NMR (CDCl 3 ): δ1.16(d, J=6.3Hz, 12H), δ3.198~3.26(q, 4H), δ3.372(d, J=2.41Hz, 8H), δ3.387(s, 12H), δ3.985~4.032 (m, 8H), δ4.865 (s, 2H).
[0032] 13 ...
Embodiment 3
[0034] Soak the 732 resin in distilled water for 24 hours, then soak it in 2mol / L HCl solution for 24 hours, wash it repeatedly with distilled water until the pH value does not change, filter it and dry it in an oven.
[0035] Add 3g of tetramethoxymethyl glycoluril, 10g of dried 732 resin, and 60g of PGME into a 250ml three-neck flask, heat up to 60°C, stir and react at -0.08MPa pressure for 15 hours, and distill off the generated methanol at the same time.
[0036] The reactant was suction-filtered with a Buchner funnel, and the filter residue was washed twice with PGME to recover the resin. The filtrate and washing liquid were combined, and the solvent PGME was recovered by distillation under reduced pressure to obtain 5.07 g of 1,3,4,6-tetrakis(1'-methyl-2'-methoxy)ethoxymethyl glycoluril, with a yield of 97.8% .
[0037] Measured product ESI-MS (m / z): 551.6 (M+1).
[0038] IR(KBr)v / cm -1 : 2929, 1714, 1479, 1238, 1091, 1031, 790.
[0039] 1 H-NMR (CDCl 3): δ1.14(d, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com