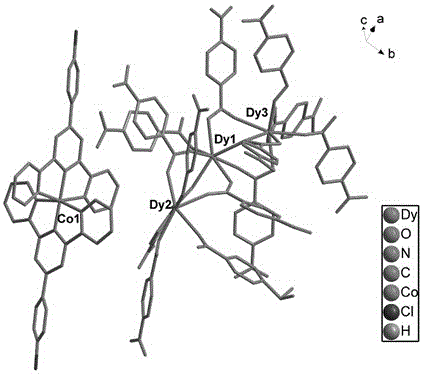
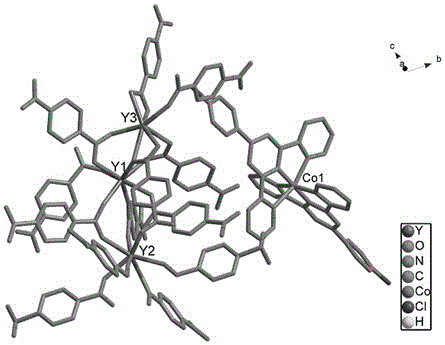
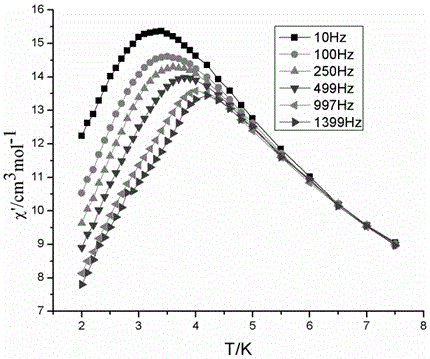
Bifunctional molecular magnet material and its synthesis method
A technology of bifunctional molecules and synthetic methods, applied in the field of preparation of molecular magnetic materials, can solve problems such as stagnation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0051] Example 1 The best reaction molar ratio selection
[0052] According to the synthesis method of the above-mentioned bifunctional molecular magnet, select CoCl 2 •6H 2 O, 4'-(4-chlorophenyl)-2,2':6',2''-terpyridine ligand, Dy 2 o 3 and p-nitrobenzoic acid were weighed at the molar ratios of 1:2:1:8, 1:1:1:8, and 2:1:1:8, respectively, and reacted at 180°C for 4320min. After the reaction, the products obtained under three different ratios were observed under a microscope:
[0053] Purple-red crystals were all precipitated. Compared with the results, the crystals prepared at the ratio of 1:2:1:8 had the best quality, large size, high purity, and high yield (respectively 70%, 55%, and 38%). In summary, this example shows that the optimal reaction molar ratio for the preparation of bifunctional molecular magnets is 1:2:1:8.
example 2
[0054] The selection of example 2 optimal reaction temperature
[0055] According to example 1, select the optimal reaction molar ratio CoCl 2 •6H 2 O: 4'-(4-chlorophenyl)-2,2':6',2''-terpyridine Ligand: Dy 2 o 3 : p-nitrobenzoic acid=1:2:1:8, choose different reaction temperatures 160°C, 180°C, 200°C respectively, and react for 3 days. Colorless transparent needle-like crystals (precipitation of the ligand), the product is not pure; the products obtained at 180°C are all large purple-red crystals, which are phase-pure; at 200°C, the reaction purple-red crystals are small, and there are black impurities on the crystal surface. Comparing the obtained results, 180°C is the optimum reaction temperature of the bifunctional molecular magnet.
example 3
[0056] Example 3 Selection of Optimal Response Time
[0057] According to examples 1 and 2, choose the best reaction ratio 1:2:1:8, the best reaction temperature is 180°C, and choose different reaction times: 2 days, 3 days, and 4 days, and it is found that the crystals obtained after 2 days of reaction The crystals were small and the yield was low at 40%; the crystals obtained after 3 days of reaction were large and very pure with a yield of 70%; after 4 days of reaction, the resulting product was charred, most of which were twins, and could not be tested. Comparing the results, the best response time is 3 days.
[0058] Based on examples 1, 2, and 3, the best preparation condition for bifunctional molecular magnets is CoCl 2 •6H 2 O: 4'-(4-chlorophenyl)-2,2':6',2''-terpyridine Ligand: Dy 2 o 3 : Under the condition of p-nitrobenzoic acid=1:2:1:8, react at 180°C for 3 days.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com