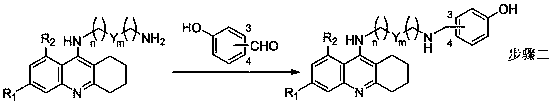
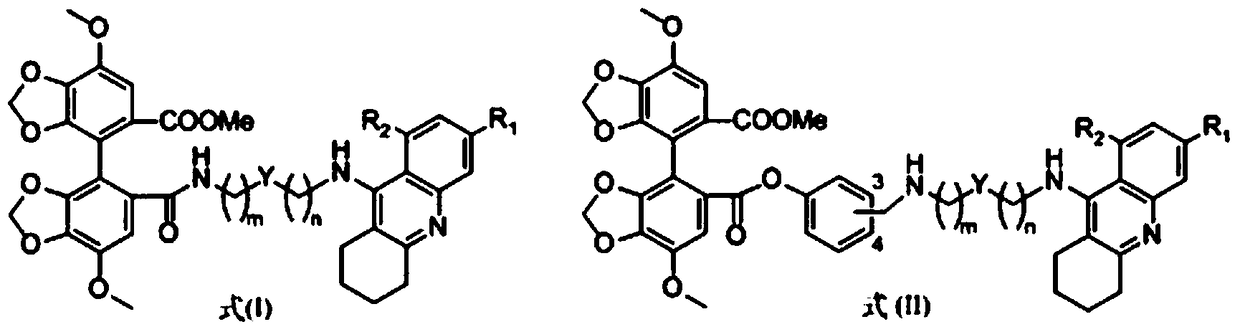
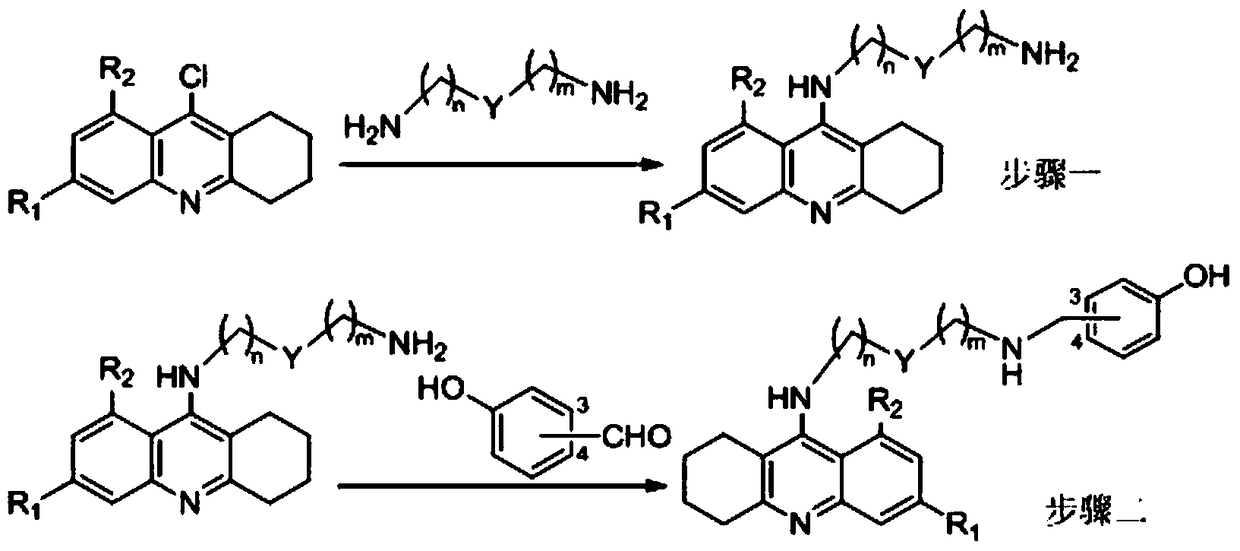
Tacrine-bifendate hybrid compound, its preparation method and application
A biphenyl diester and hybrid technology, which is applied in the field of tacrine-biphenyl diester hybrid, can solve the problems of poor human body tolerance, poor water solubility, retention and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
[0038] Methyl-7,7'-dimethoxy-5'-(4-(1,2,3,4-tetrahydroacridin-9-amino)butylcarboxamido)-4,4'-diphenyl [d][1,3]dioxymethylene-5-carboxylate.
[0039] Will N 1 -(1,2,3,4-tetrahydroacridine-9-amino)butyl-1,4-butanediamine (1.0mmol), biphenyl diester acid chloride (1.0mmol), triethylamine (0.5mL ) was dissolved in 20mL of chloroform, stirred at room temperature for 12 hours, after the reaction was completed, the solvent was evaporated to dryness, 30mL of ethyl acetate was added, washed three times with water, and the organic layer was washed with anhydrous Na 2 SO 4 Dry, evaporate the solvent to dryness, and separate by silica gel column chromatography. The eluent is chloroform:methanol:ammonia=200:5:1 (volume ratio).
[0040] The product is light yellow solid, yield: 61%, 1 H NMR (400MHz, CDCl 3 )δ7.90(d, J=9.6Hz, 2H), 7.51(t, J=8.2Hz, 1H), 7.31(t, J=8.2Hz, 1H), 7.17(s, 1H), 7.00(s, 1H), 6.27(t, J=5.9Hz, 1H), 5.94(d, J=1.1Hz, 1H), 5.91(d, J=1.1Hz, 1H), 5.89(dd,...
Embodiment 2
[0042]
[0043] Methyl-7,7'-dimethoxy-5'-(6-(1,2,3,4-tetrahydroacridin-9-amino)hexylcarboxamido)-4,4'-di Phenyl[d][1,3]dioxymethylene-5-carboxylate.
[0044] Method is the same as embodiment one, and difference is to use N 1 -(1,2,3,4-tetrahydroacridin-9-amino)hexyl-1,6-hexanediamine instead of N 1 -(1,2,3,4-tetrahydroacridine-9-amino)butyl-1,4-butanediamine, finally a light yellow solid was obtained.
[0045] Yield: 41%, 1 H NMR (400MHz, CDCl 3 )δ7.99(dd, J=12.4,8.4Hz,2H),7.55(t,J=8.1Hz,1H),7.35(t,J=7.7Hz,1H),7.23(s,1H),7.05( s,1H),6.21(t,J=5.9Hz,1H),5.97(d,J=7.6Hz,2H),5.92(s,2H),4.47(s,1H),3.92(s,3H), 3.90(s,3H),3.76(s,3H),3.51(t,J=6.9Hz,2H),3.32(dd,J=13.5,6.8Hz,1H),3.11-2.99(m,3H),2.70 (s,2H),1.90(s,4H),1.65-1.55(m,2H),1.32-1.17(m,4H),1.13-1.03(m,2H). 13 C NMR (100MHz, CDCl 3 )δ167.95,167.41,157.18,151.57,147.90,146.45,145.93,143.27,142.82,138.48,136.20,130.70,128.93,127.27,124.45,123.85,123.08,119.49,115.13,110.76,110.69,108.31,107.89,102.63,102.08 ,56.74,56.45...
Embodiment 3
[0047]
[0048] Methyl-7,7'-dimethoxy-5'-(8-(1,2,3,4-tetrahydroacridin-9-amino)octylcarboxamido)-4,4'- Diphenyl[d][1,3]dioxymethylene-5-carboxylate.
[0049] Method is the same as embodiment one, and difference is to use N 1 -(1,2,3,4-tetrahydroacridine-9-amino)octyl-1,8-octanediamine instead of N 1 -(1,2,3,4-tetrahydroacridine-9-amino)butyl-1,4-butanediamine, finally a light yellow solid was obtained.
[0050] Yield: 66%, 1 H NMR (400MHz, CDCl 3 )δ7.98(d, J=8.0Hz, 1H), 7.93(d, J=8.4Hz, 1H), 7.55(t, J=7.1Hz, 1H), 7.34(t, J=7.6Hz, 1H) ,7.23(s,1H),7.06(s,1H),6.11(t,J=5.8Hz,1H),5.98(dd,J=8.4,1.1Hz,2H),5.92(s,2H),4.17( s,1H),3.93(d,J=2.0Hz,6H),3.76(s,3H),3.50(t,J=7.2Hz,2H),3.31(dq,J=13.6,6.9Hz,1H), 3.10-2.97(m,3H),2.70(s,2H),1.93-1.90(m,4H),1.69-1.60(m,2H),1.40-1.31(m,2H),1.26-1.14(m,6H ),1.07-0.97(m,2H). 13C NMR (100MHz, CDCl 3 )δ167.87,167.42,157.94,151.11,147.92,146.87,146.42,143.31,142.85,138.51,136.19,130.78,128.54,128.18,124.42,123.69,122.95,119.94,115.54,110.75,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com