Application of zinc finger protein 436 (ZNF 436) to treatment of myocardial hypertrophy
A technology of zinc finger protein and cardiac hypertrophy, applied in the application of drugs for alleviating and/or treating cardiac hypertrophy, preparation of prevention, application field of zinc finger protein 436 (ZNF436) in the treatment of cardiac hypertrophy, which can solve the problem of no therapeutic drugs and In order to achieve the effects of inhibiting cardiac hypertrophy, anti-cardiac fibrosis, cardiac hypertrophy and protecting cardiac function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] [Example 1] Expression of ZNF436 in the hearts of normal people and patients with dilated heart disease
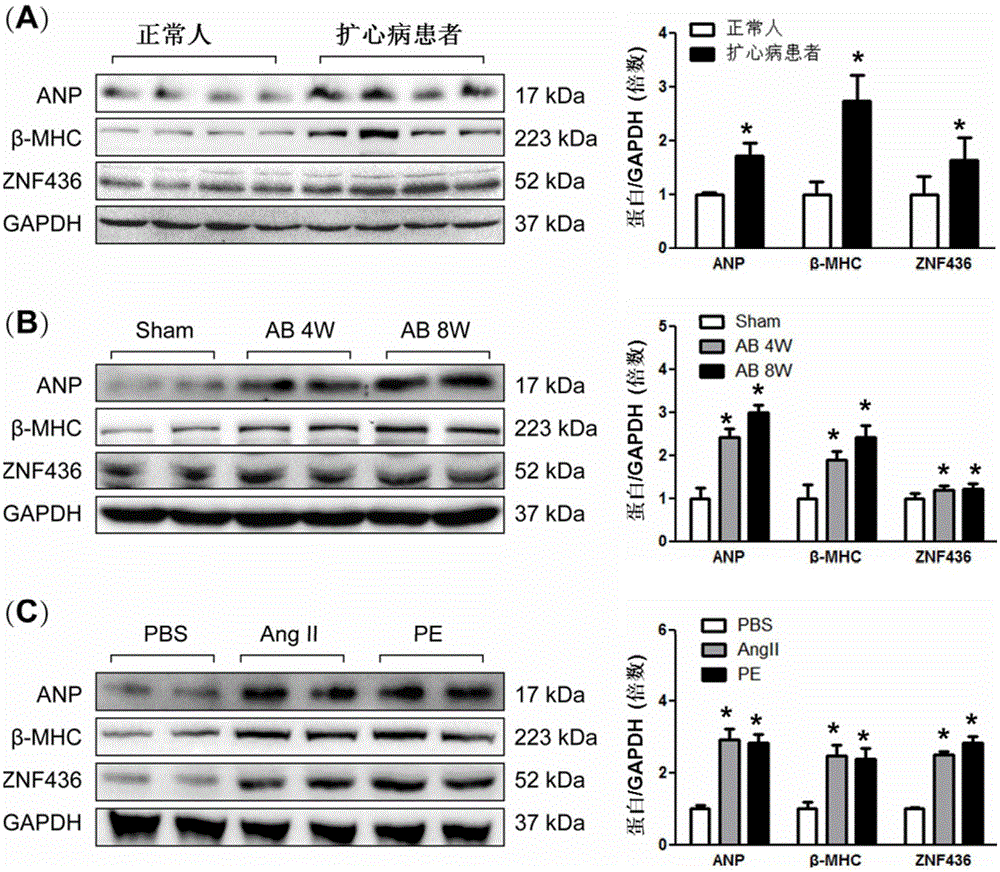
[0084] Normal human hearts (individuals donated by non-cardiac causes of death) and hearts of patients with dilated heart disease (recipients replaced by patients undergoing heart transplantation) were selected, and SDS-PAGE-Western blot test (Western blot) was performed on the proteins extracted from the hearts, and the binding specificity Antibodies recognizing ZNF436 protein and cardiomyocyte hypertrophy markers ANP (Millipore, AB2232) and β-MHC (santacruz, sc53090) were detected to measure the expression of ZNF436 (ATLAS, HPA043817), and GAPDH (Cell Signaling Technology, 2128) was used as an internal reference. Test results such as figure 1 As shown in A, the expression of cardiomyocyte hypertrophy markers ANP and β-MHC in the heart of patients with dilated cardiomyopathy was significantly up-regulated, and the expression of ZNF436 was significantly up-regulated...
Embodiment 2
[0085] [Example 2] Expression of ZNF436 in wild-type mouse Sham group and AB operation 4W, 8W hearts
[0086] 1. The myocardial hypertrophy model adopts aortic arch coarctation surgery, and the operation process of the model is as follows:
[0087] 1.1 Preoperative preparation
[0088] (1) Anesthesia: First weigh the mice, calculate the required amount of anesthetic (3% pentobarbital sodium) according to 90 mg / kg body weight, inject intraperitoneally, and record the injection time point. There is no obvious reaction between tail and toe pinching and the mouse is in good condition. This is the standard for successful anesthesia (generally there is no obvious reaction about 10 minutes after injection, and the mouse has a reaction to pinch toe about 50 minutes after anesthesia, and about 30 minutes after anesthesia is the best operation time).
[0089] (2) Preparation of the operation area: the skin of the left chest, left chest and armpit of the left forelimb of the mouse was r...
Embodiment 3
[0099] [Example 3] Expression of ZNF436 in cardiomyocytes stimulated by control group (PBS) or angiotensin II (AngII) or phenylephrine (PE)
[0100] Isolate and culture newborn 1-day Sprague-Dawley neonatal rat cardiomyocytes, culture the primary cardiomyocytes for 48 hours, change the medium, add serum-free DMEM / F12 starved cardiomyocytes for 12 hours to synchronize the cells, and give PBS and angiotensin II (AngII respectively) , 1 μM), phenylephrine (PE, 1 μM) stimulation for 48 hours, SDS-PAGE-immunoblotting test (Western blot) was performed on the protein extracted from cardiomyocytes, combined with specific recognition of ZNF436 protein and cardiomyocyte hypertrophy markers ANP, β-MHC The antibody was detected, and the expression of ZNF436 was determined, and the detection results were as follows: figure 1 C shown. The expressions of ANP, β-MHC and ZNF436 were significantly up-regulated in cardiomyocytes stimulated by angiotensin II (AngII) or phenylephrine (PE).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com