Application of Chenopodolin in preparation of medicines for treating ovarian cancer
A kind of chenopodolin, 1. The technology of chenopodolin, which is applied in the application field of chenopodolin in the preparation of drugs for treating ovarian cancer, can solve the problems that there is no report of ovarian cancer activity, no antibacterial or antifungal activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Example 1: Isolation, Preparation and Structure Confirmation of Chenopodolin
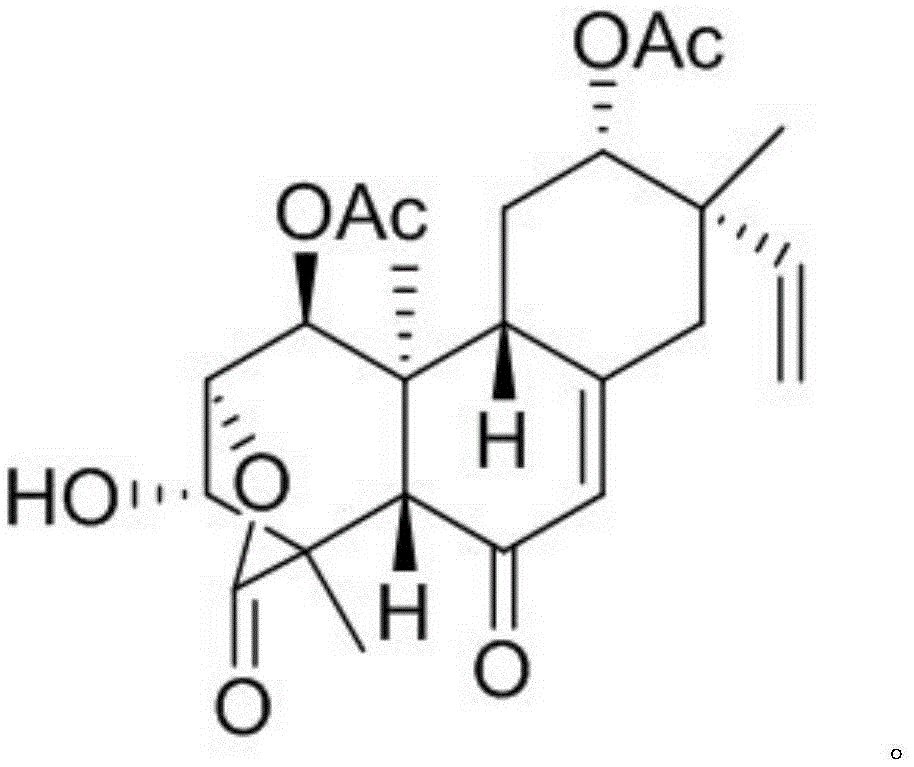
[0011] The preparation method of Chenopodolin is the same as that reported in the literature (Chenopodolin: APhytotoxic Unrearranged ent-Pimaradiene Diterpene Produced by Phomachenopodicola, a Fungal Pathogen for Chenopodium album Biocontrol, J. Nat. Prod., 2013, 76, 1291-1297).
[0012] Structural confirmation: the molecular formula is C 24 h 30 o 8 , with an unsaturation of 10. H NMR spectrum data δ H (ppm, DMSO-d 6 , 600MHz): H-1 (5.00, d, J=4.8), H-2 (4.62, br, d, J=4.8), H-3 (4.28, br, s), H-5 (2.74, s ), H-7 (5.85, s), H-9 (2.85, m), H-11 (1.50, m), H-12 (4.56, dd, J=10.0, 4.1), H-14 (2.62, d, J=16.2), H-14 (2.26, d, J=16.2), H-15 (5.79, dd, J=17.8, 11.0), H-16 (5.17, d, J=11.0), H- 16 (4.97, d, J=17.8), H-17 (1.06, s), H-19 (1.48, s), H-20 (1.08, s), 1-COMe (2.24, s), 12-COMe (2.12, s); carbon NMR spectrum data δ C (ppm, DMSO-d 6 , 600Hz): 71.8 (CH, 1-C), 79.3 (CH, 2-C), 76.8...
Embodiment 2
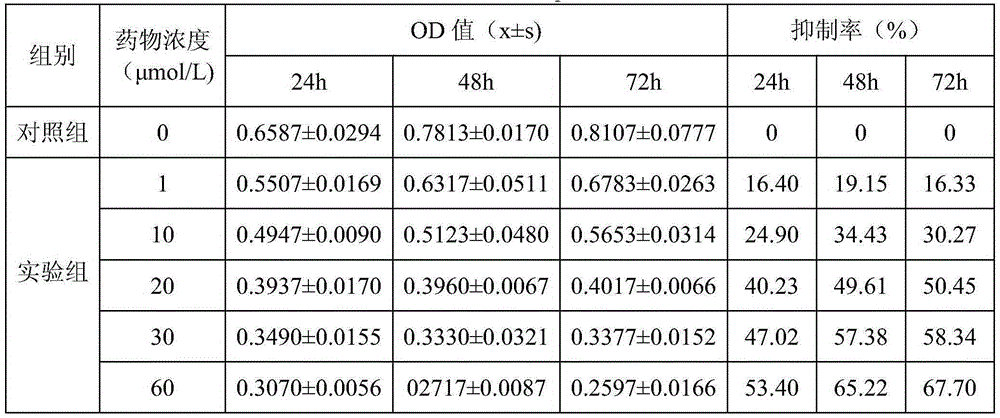
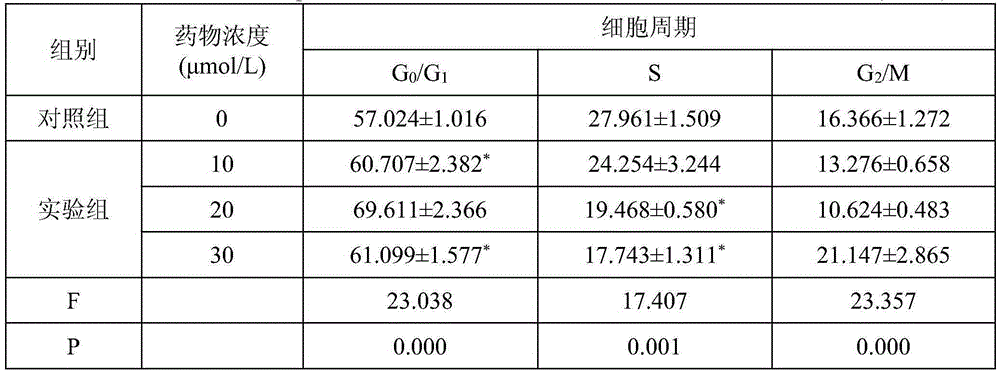
[0013] Embodiment 2: Pharmacological action test of Chenopodolin
[0014] 1. Materials and Instruments
[0015] The human ovarian serous papillary cystadenocarcinoma cell line SKOV3 was provided by the Medical Experimental Center of Lanzhou University. Common RPMI-1640 medium (10% fetal bovine serum) culture. Chenopodolin is self-made, and the HPLC normalized purity is greater than 98%. RPMI-1640 culture medium, tetramethylazoblue (MTT), dimethyl sulfoxide (DMSO), L-glutamine Kehao Bioengineering Co., Ltd. Trypsin was purchased from Sigma, USA. Fetal bovine serum was purchased from Hangzhou Sijiqing Bioengineering Materials Co., Ltd. Sodium dodecyl sulfonate (SDS) was purchased from Xi'an Zhouding Biotechnology Co., Ltd. HER2 (FITC marker) was purchased from Beijing Boaosen Biotechnology Co., Ltd. Acidified HER2 (FITC-labeled) was purchased from Beijing Boaosen Biotechnology Co., Ltd. Penicillin and streptomycin were purchased from Huabei Pharmaceutical Factory.
[001...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com