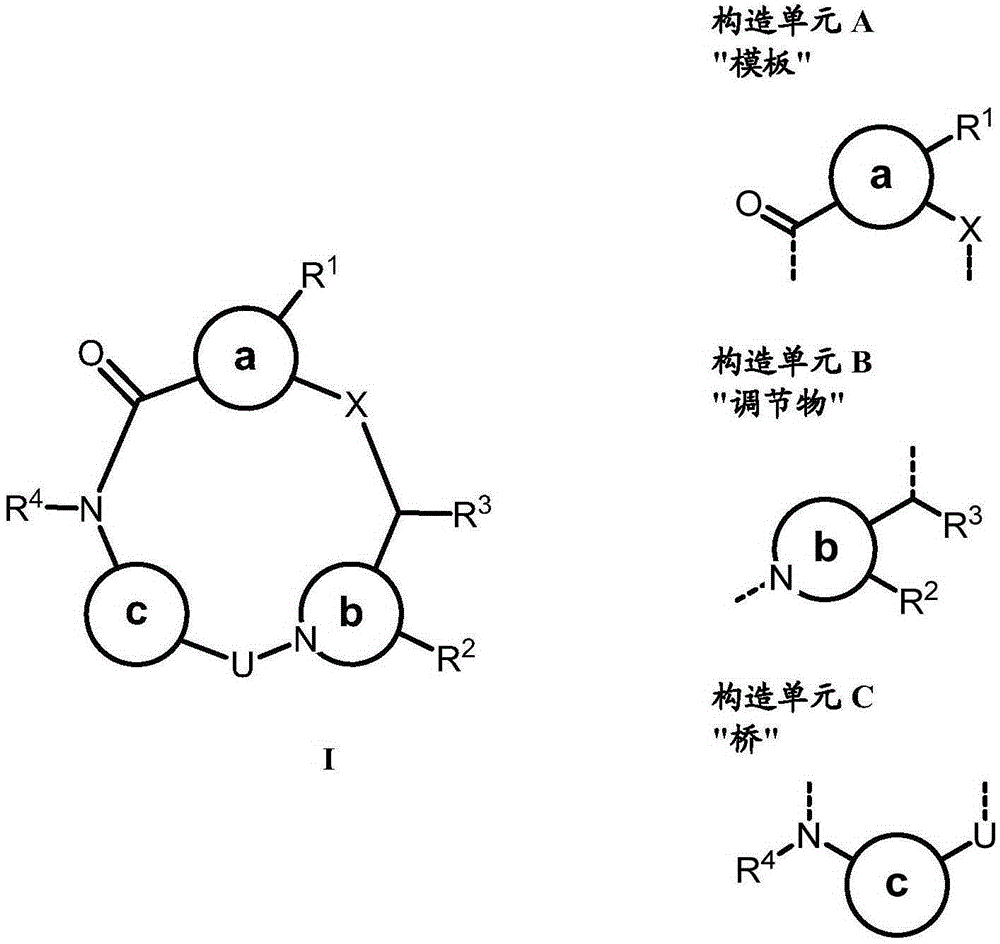
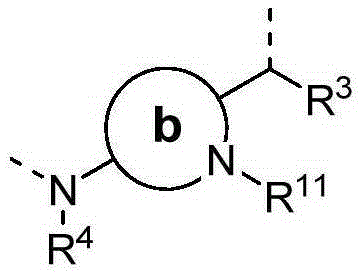
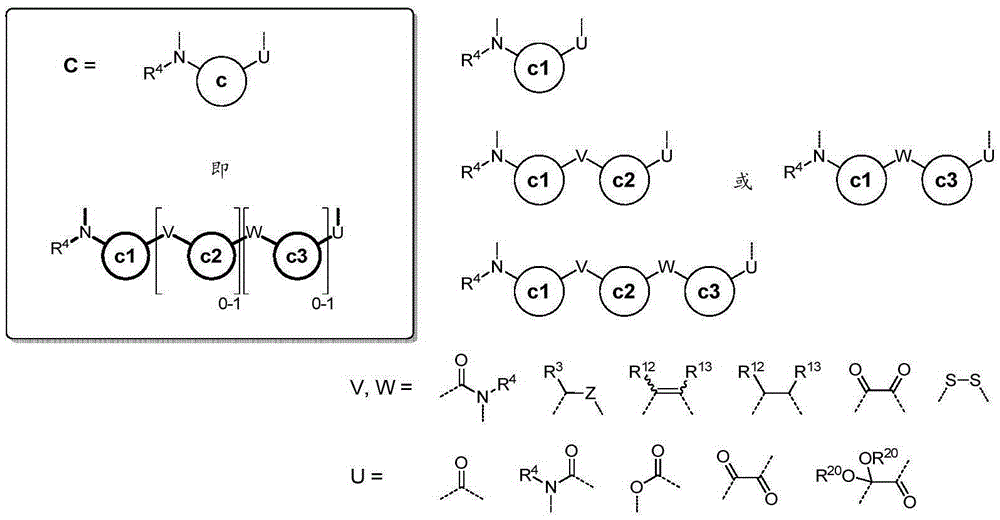
Conformation-constrained entirely-synthetic macrocyclic compounds
A compound and construction technology, applied in the field of conformationally restricted fully synthesized macrocyclic compounds, can solve complex quality control and development process problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0942] Data of Example 1: C 31 H 40 FN 3 O 7 Si (613.7). LC-MS (Method 7):
[0943] R t =1.45(41),614([M+H] + ); 1.47(44), 614([M+H] + ).
[0944] 1 H-NMR(DMSO-d 6 ): complex spectrum, several isomers; 7.45-7.01 (m, 8H), 6,78-6.58 (2m, 1H), 5.42-5.06 (m, 3H), 4.50-3.50 (several m, 7H) , 3.30-1.40 (several kinds of m, 7H), 2.84, 2.70, 2.66 (s, 3H), 0.97-0.82 (m, 2H), 0.03, 0.02, 0.00 (s9H).
Embodiment 2
[0945] Core 02: Synthesis Example 2 (Scheme 11)
[0946] Example 2 Synthesis of Protected Macrolide
[0947] In 2 hours, T3P (50%, EtOAc, 0.75mL, 1.27mmol) and iso-Pr 2 NEt (0.36mL, 2.2mmol) anhydrous CH 2 Cl 2 (20mL) Add amino acid 98 (250mg, 0.43mmol) in anhydrous CH to the solution 2 Cl 2 (730 mL) solution. Stir the solution at room temperature for 20 hours, then use saturated Na 2 CO 3 Aqueous extraction. Dry organic phase (Na 2 SO 4 ), filter and concentrate. FC(CH 2 Cl 2 / MeOH100:0 to 95:5), providing Example 2 (187 mg, 77%).
[0948] Data of Example 2: C 30 H 36 FN 3 O 7 (569.6)LC-MS (Method 7):
[0949] R t =1.35(62),570([M+H] + ); 1.39(15), 570([M+H] + )
[0950] 1 H-NMR(DMSO-d 6 ): complex spectrum, several isomers; 7.46-7.30 (m, 5H), 7.27-7.06 (m, 2H), 6.98-6.67 (4dd, 1H), 5.54-5.06 (m, 3H), 4.68-3.48 (m, 6H), 3.05-1.98 (m10H; s at 2.82, 2.69, 2.64), 1.44-1.41 (3s, 9H).
[0951] Core 03: Synthesis Example 3, Example 4, and Example 5 (Scheme 7)
[0952] Synthesis of Mitsunob...
Embodiment 3
[0959] Data of Example 3: C 33 H 41 FN 4 O 8 (640.7). HPLC(30%CH 3 CN): R t = 3.20 (96). LC-MS (Method 9c): R t =2.06,641([M+H] + ). 1 H-NMR(CDCl 3 ): 7.45-7.32 (m, 5H), 7.06 (m, 1H), 6.94-6.88 (m, 2H), 5.57 (dd, J=2.8, 12.6, 1H), 5.42 (width m, 1H), 5.26 ( d, J = 12.2, 1H), 5.15 (d, J = 12.2, 1H), 4.90 (dd, J = 2.5, 11.0, 1H), 4.34 (d, J = 17.2, 1H), 4.35 to 4.11 (m, 3H), 3.82 (width t, J about 8.5, 1H), 3.65 (d, J=17.3, 1H), 3.29 (t, J about 8.8, 1H), 3.14 (s, 3H), 2.65 (s, 3H) , 2.51-1.98 (several kinds of m, 5H), 1.76 (td, J=8.2, 12.7, 1H), 1.36 (s, 9H).
[0960] Synthesis acid example 4:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com