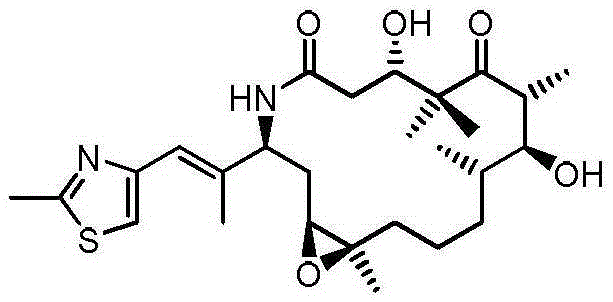
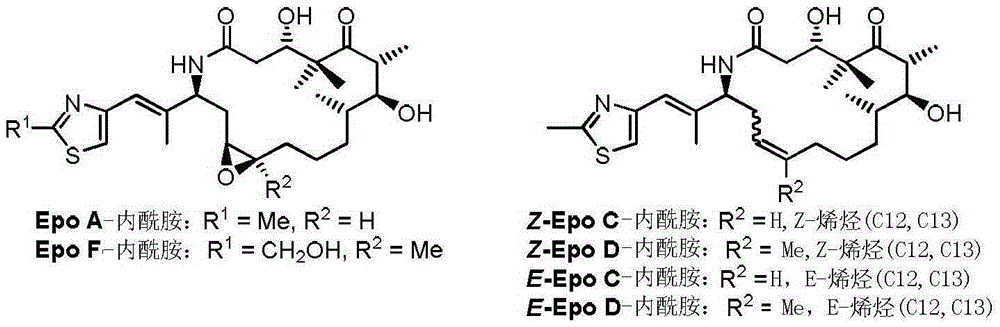
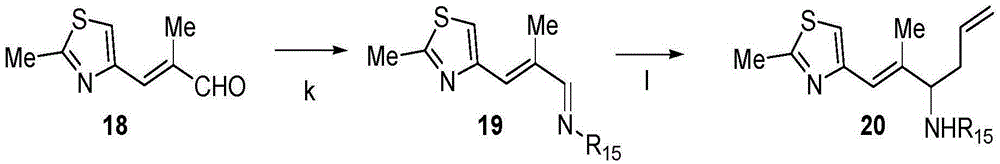
Process for ixabepilone, and intermediates thereof
一种选自、化合物的技术,应用在有机化学等方向,能够解决昂贵负担、重大等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0156] Unlike other routes for the preparation of ixabepilone, the method described herein does not require triphenylarsenic (AsPh 3 )The presence. Triphenylarsenic is an organic compound of arsenic, and arsenic is known to be toxic. Unexpectedly, in the described embodiments, a rapid and efficient Suzuki reaction occurs in the absence of arsenic-containing catalysts. This result is particularly beneficial for the safety of production personnel and the management of waste associated with avoiding arsenic-containing toxic chemicals.
[0157] Furthermore, the Suzuki reaction described herein coupling compound II and compound III to provide product IV gave higher yields than the method described in J. Org. Chem. 2001, 66, 4369-4378. For example, when N-BOC-protected amine IIIa' is coupled with IIax', >90% yields can usually be obtained. In contrast, the yield of the coupling of N-BOC-protected amine IIIa' to compound D2a reported in J.Org.Chem. 2001, 66, 4369-4378 was only 10%...
Embodiment 1
[0310] Example 1 - Preparation of XIXa and XIXa'
[0311] Preparation of compound XVIII'
[0312]
[0313] in N 2 To a solution of compound XVI' (100 g, 389 mmol) in anhydrous DCM (800 mL) was added dropwise Et at room temperature under atmosphere. 3 N (73 mL, 506 mmol) and TBSOTf (113 g, 428 mmol). The resulting mixture was stirred overnight at room temperature (Solution A). In another flask, under N 2 To a solution of XVII (80 g, 622 mmol) in anhydrous DCM (500 mL) was added TiCl at -78 °C under atmosphere 4 (IN in DCM, 650 mL, 650 mmol). After stirring at -78°C for 10 minutes, solvent A was added dropwise to XVII over about 1 hour, and the resulting mixture was allowed to warm to room temperature and then stirred overnight. After the completion of the reaction was demonstrated by TLC, the reaction mixture was washed with saturated NH 4 Aqueous Cl solution was quenched, and the aqueous layer was extracted with DCM (400 mL×2). The combined organic layers were washe...
Embodiment 2
[0329] Example 2 - Preparation of XIVax
[0330] Preparation of XIa
[0331]
[0332] in N 2 To a solution of C-C29a (35 g, 200 mmol, Rf=0.5, EtOAc:petroleum ether 1:3, UV) in anhydrous THF (414 mL) was added (R)-tert-butylsulfinate under atmosphere and at room temperature Amide (29g, 400mmol) and Ti(i-PrO) 4 (118 mL, 400 mmol), then the reaction mixture was stirred overnight. The mixture was cooled to 5°C with an ice bath and brine (150 mL) was added carefully. The resulting suspension was diluted with EtOAc (100 mL), filtered through a pad of diatomaceous earth, and the filter cake was washed twice with EtOAc (150 mL). The filtrate was washed with brine (3x260mL), washed with Na 2 SO 4 Dry, filter and concentrate under reduced pressure. The residue was purified by column chromatography (silica gel, EtOAc:petroleum ether=1:5) to yield XIa (42 g, yield: 77%, Rf=0.4, EtOAc:petroleum ether=1:3, UV) as a yellow solid. 1 HNMR (400MHz, CDCl 3 )δ8.25(s,1H),7.31(s,1H),7.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com