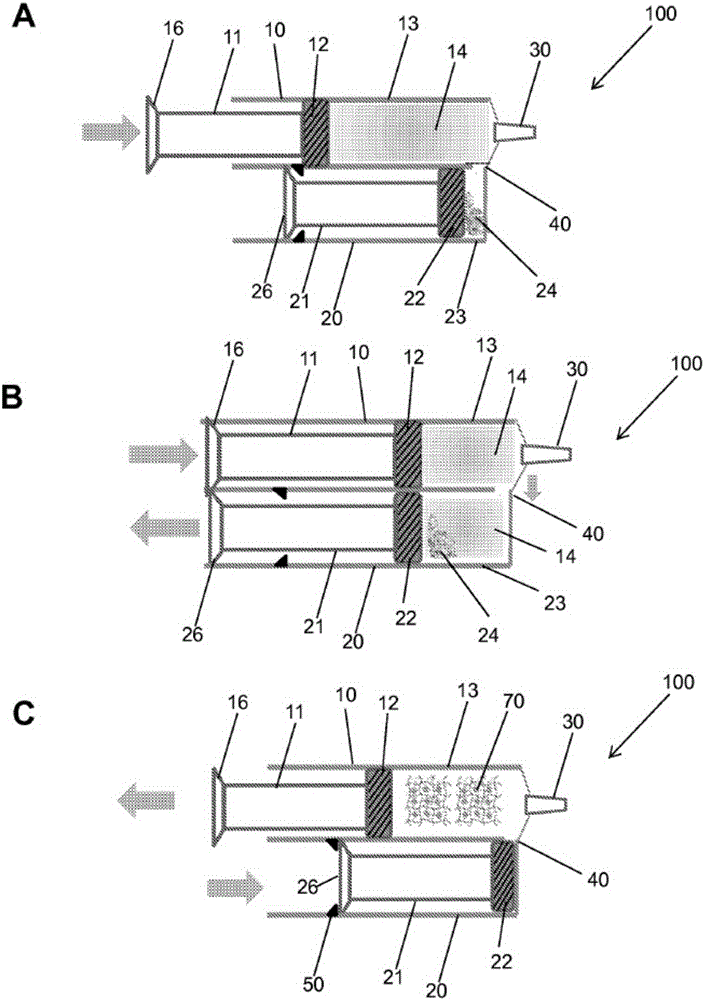
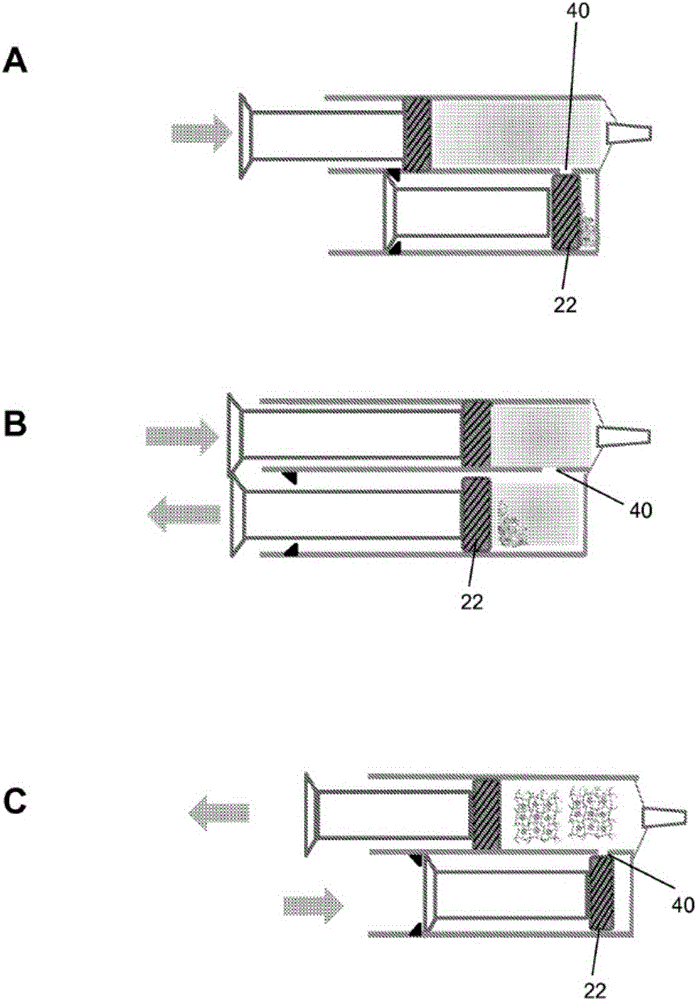
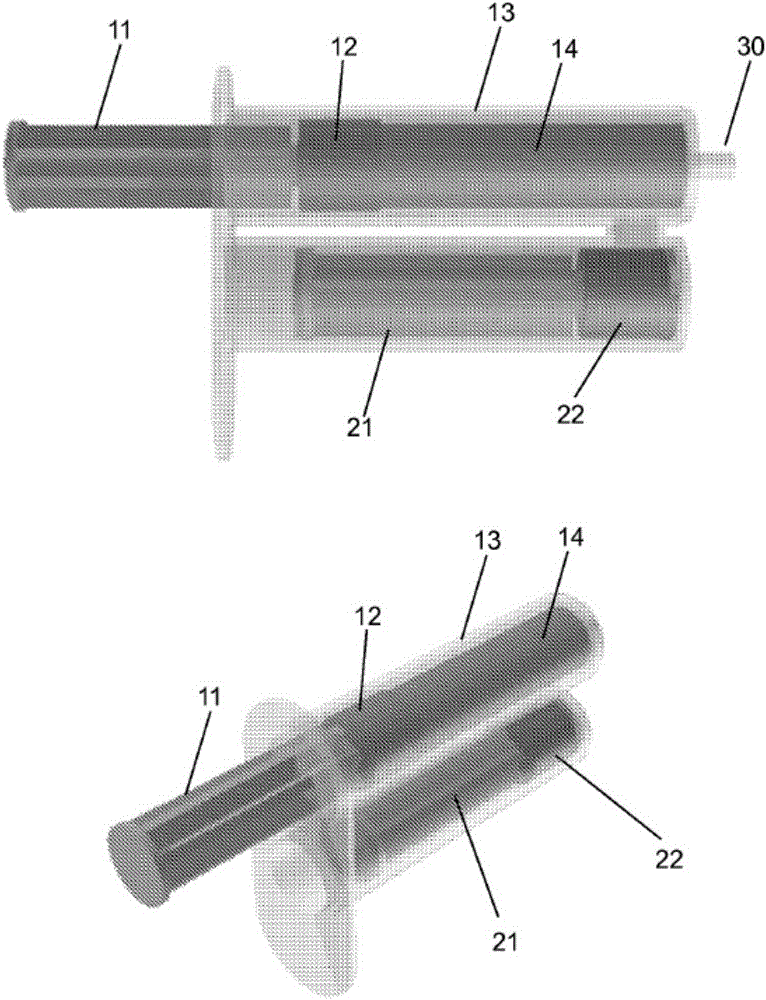
Hydrogel pressure sealant system
A technology of hydrogel and therapeutic agent, applied in the field of hydrogel pressure sealant system, which can solve the problems of pneumothorax
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0094] Example 1: DCH and Gelatin Hydrogels with Lidocaine
[0095] Lidocaine-containing amphiphilic diblock copolypeptide hydrogels (DCH) were fabricated using different concentrations of K180L20 and compared with hydrogels fabricated with gelatin. These gels were formulated using saline buffer. Figure 7 Three compositions depicting hydrogels: left: 3% K180L20 + 1% lidocaine (99.7pa); middle: 4% K180L20 + 1% lidocaine (170.7pa); right: 10% gelatin + 1% Lidocaine (233Pa). In vitro and in vivo experiments with hydrogel compositions were performed to determine the optimal composition to provide the desired level of stiffness, tissue adhesion, pressure, sealing ability and lidocaine release rate for lung needle biopsy.
example 2
[0096] Example 2: HA Hydrogel
[0097] Hyaluronic acid (HA)-based hydrogels have demonstrated in vivo use in the past and are FDA-approved for various applications. Three concentrations of HA-based hydrogels were prepared for injection, 3% (w / v), 4% (w / v), and 5% (w / v), by mixing an appropriate amount of HA with saline buffer. The HA gel achieves the chosen stiffness. However, the presence of chemical linkages within the gel does not allow injection of the gel through a coaxial needle. HA gels tend to remain cohesive and collect in tubes ( Figure 8 ).
example 3
[0098] Example 3: Hydrogel Stiffness
[0099] The hydrogels of the present invention are required to have an optimal stiffness to allow the operator / interventionist to inject without encountering significant resistance, and the ability to stay in place without significantly spreading out from the target area. To test for proper stiffness, DCH at concentrations of 1% (w / v), 3% (w / v) and 4% (w / v) and 6% (w / v) and 10% (weight / volume) gelatin at various concentrations to subjectively determine the approximate range of available gel stiffness. Concentrations of 1% to 4% were injected without significant resistance. Higher resistance was felt at 6% and 10% concentrations and was difficult to use in clinical practice. DCH was then injected ex vivo into the fascial plane between chicken breast muscle and skin. 1% DCH exhibited rapid dispersion, while 3% and higher concentrations of DCH remained in place moderately ( Figure 9 ). From these experiments, it was concluded that 3% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com