Preparation and application of diisoindigo monomer and its benzodithiophene bistin copolymer
A technology of benzodithiophene and thiophene double tin is applied in the field of preparation of bisisoindigo monomer and benzodithiophene double tin copolymer, which can solve the problems of unfavorable industrial production, troublesome purification, high cost, and achieve good Application prospect, simple and effective synthesis method, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
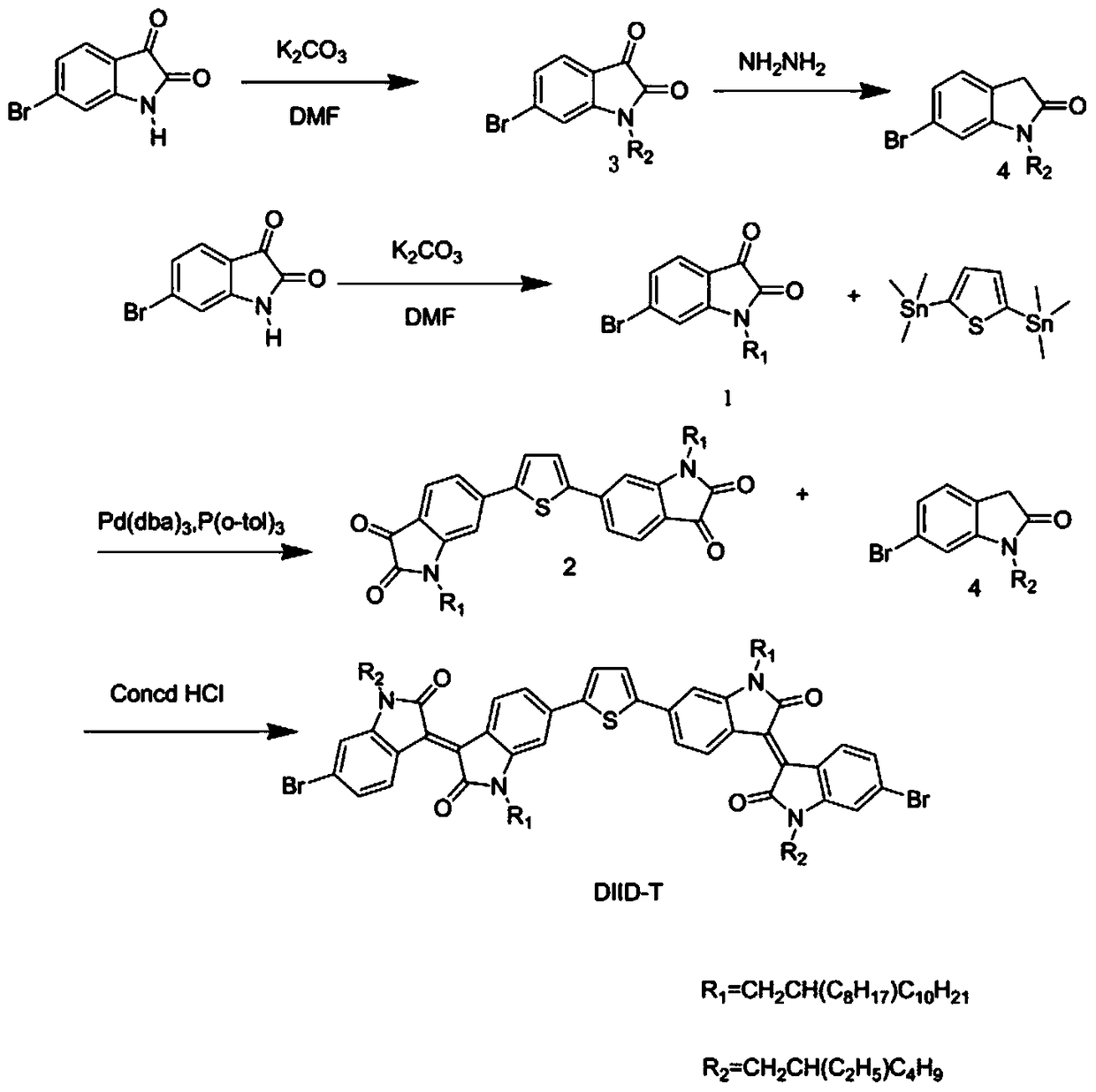
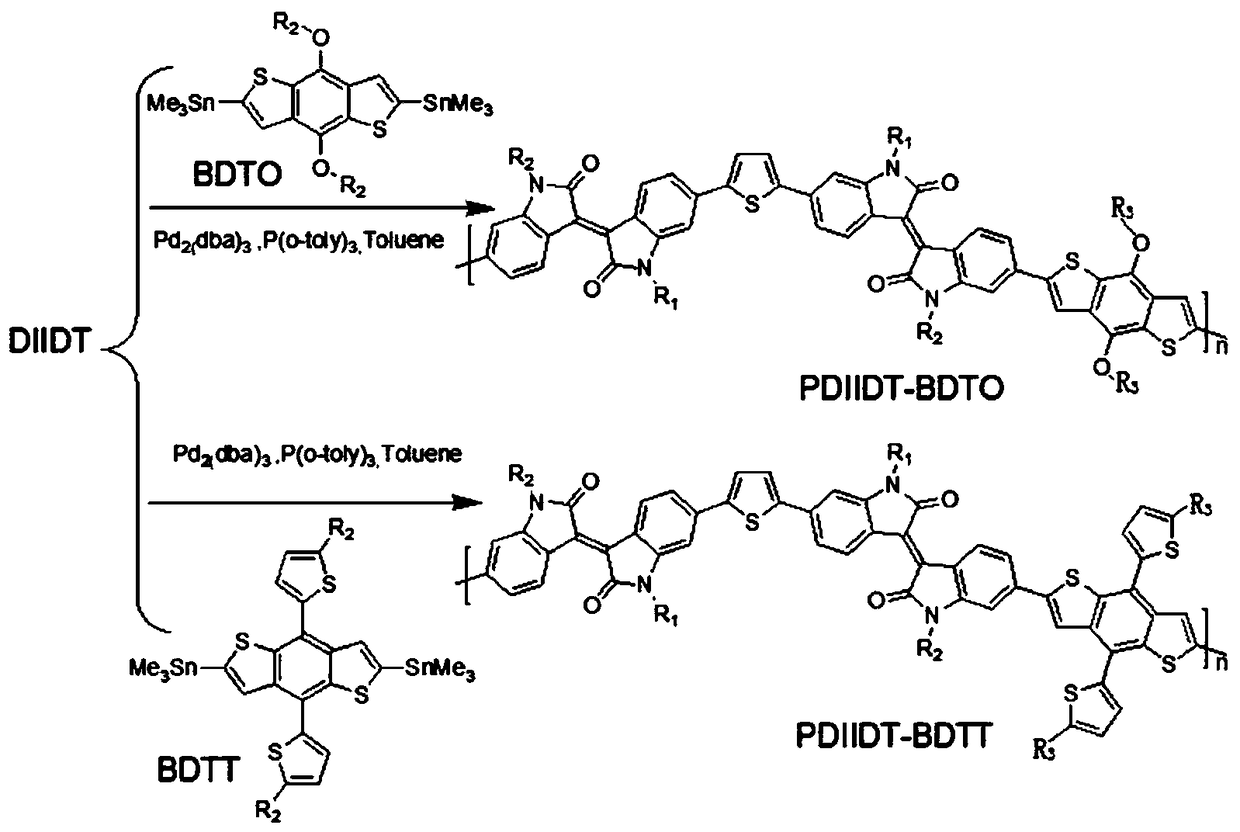
[0039] This embodiment provides a diisoindigo monomer (DIIDT) based on thiophene unit connection and its benzodithiophene bistin copolymer (PDIIDT-BDTO and PDIIDT-BDTT), its structural formula is shown in Table 1, and its single For the synthetic route of body DIDT see figure 1 , the synthesis of polymers PDIIDT-BDTO and PDIIDT-BDTT see figure 2 shown.
[0040] Table 1
[0041]
[0042] This embodiment also relates to the preparation method of polymer PDIIDT-BDTO and PDIIDT-BDTT, comprising the steps of:
[0043] (a) Synthesis of intermediate compound benzodithiophene bistin monomer
[0044] The structural formula of benzodithiophene ditin monomer is
[0045] For its detailed preparation method, see the literature "Hwang, Y.J; Kim, F.S; Xin, H; Jenekhe, S.A; New Thienothiadiazole-Based Conjugated Copolymers for Electronics and Optoelectronics. Macromolecules 2012, 45, 3732-3739; Cheng, Y.S; Chen, S.A; Fullerene Derivative-Doped Zinc Oxide Nanofilm as the Cathode of I...
Embodiment 2
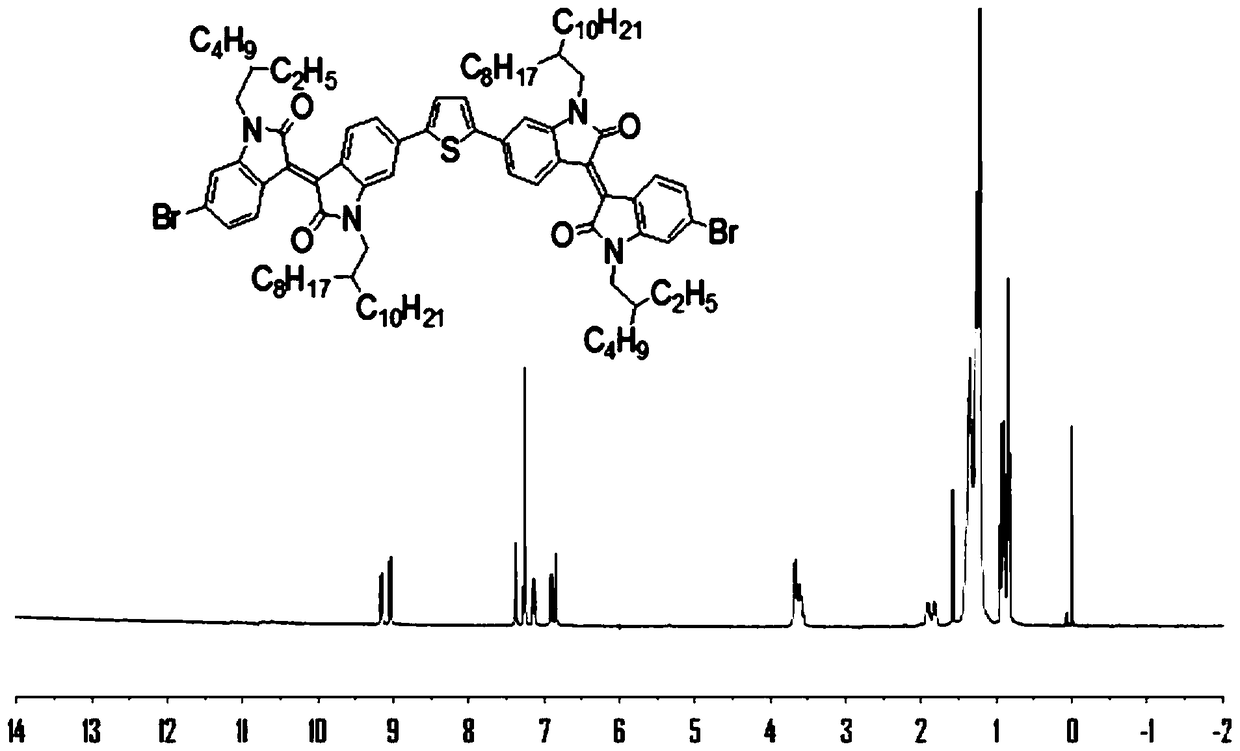
[0072] This embodiment relates to the H NMR carbon NMR spectrum of the monomer DIIDT in Example 1, the ultraviolet absorption spectrum and electrochemical properties of the polymers PDIIDT-BDTO and PDIIDT-BDTT. image 3 and Figure 4 The H NMR and C NMR spectra of monomer DIDT are given respectively. Figure 5 The UV absorption spectra of polymers PDIIDT-BDTO and PDIIDT-BDTT in chloroform are given, and the λ in chloroform solutions of PDIIDT-BDTO and PDIIDT-BDTT max abs Very close, almost coincident, respectively 695nm and 698nm; Figure 6 The solid film UV absorption spectra of the polymers PDIIDT-BDTO and PDIIDT-BDTT are given, and the UV-vis absorption of the polymer solid film λ max abs PDIIDT-BDTO 697nm, PDIIDT-BDTT 702nm, respectively red-shifted 2nm and 4nm, PDIIDT-BDTO and PDIIDT-BDTT λ onset abs are 790nm and 800nm respectively, the corresponding E g opt (Eg opt =1240 / λ onset abs ) are 1.57eV and 1.55eV, respectively. Figure 7 The cyclic voltammetry cu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com