A kind of method using laccase to catalyze the synthesis of silibinin
A technology of silibinin and laccase, which is applied to fermentation and other directions, can solve problems such as long reaction time, and achieve the effects of simple and easy-to-control reaction, simple product separation and purification, and wide temperature range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
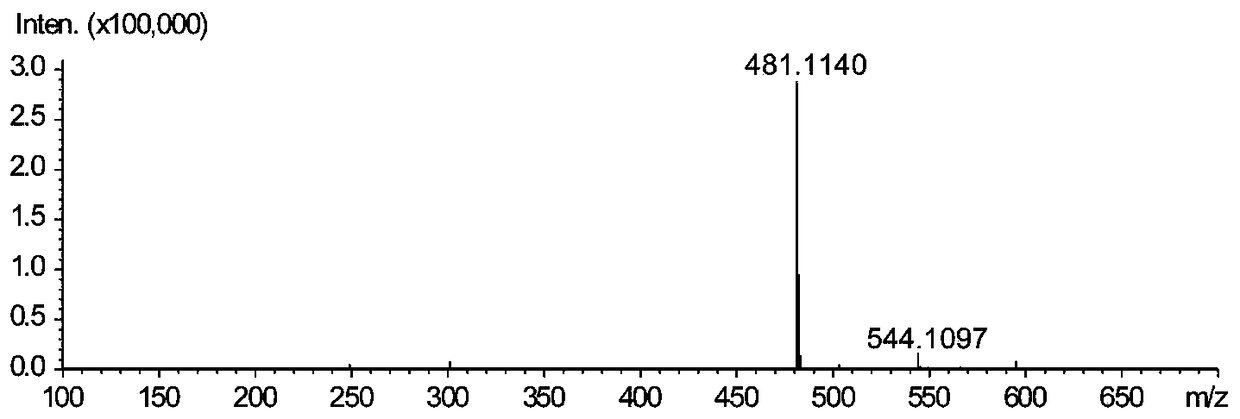
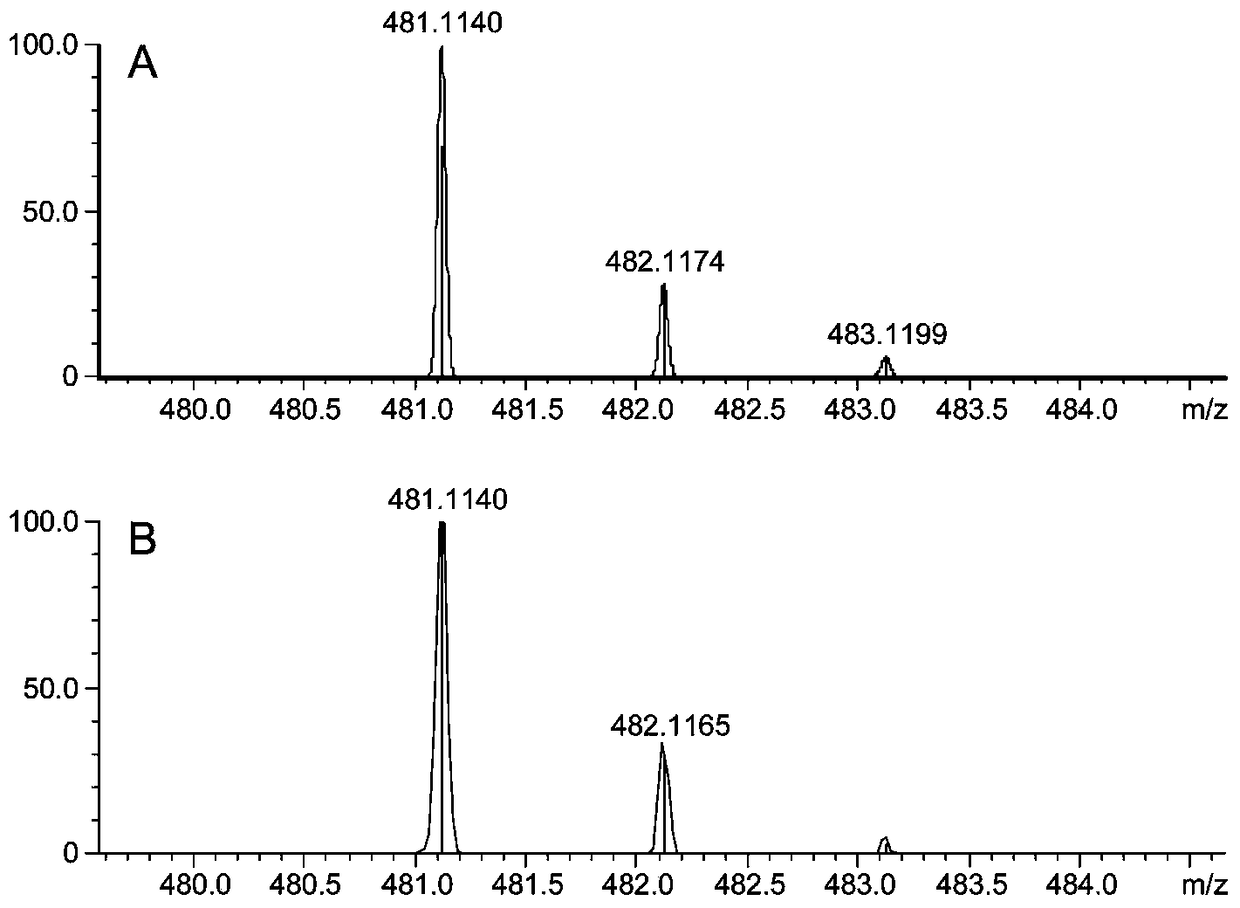
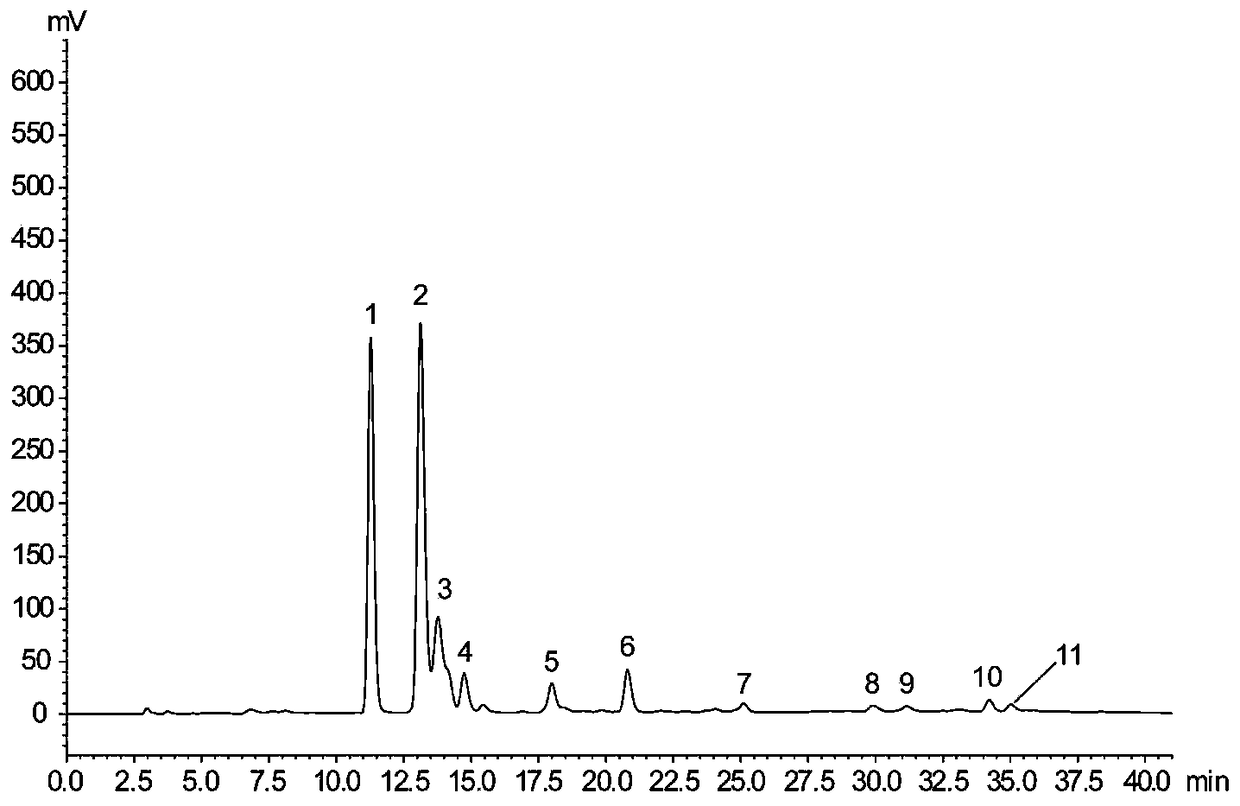
[0033] In order to verify the ability of laccase to catalyze the synthesis of silibinin from coniferyl alcohol and docetaxel, the synthesis of silibinin was catalyzed by laccase with coniferyl alcohol and docetaxel as substrates. The production of silibinin was verified when the substrate or enzyme was omitted, as shown in Table 1.
[0034] Accurately weigh a certain amount of coniferyl alcohol and docetaxel, dissolve them in 0.2M PBS (pH6.0) and adjust the volume to 0.555mM and 0.2774mM respectively. Laccase was dissolved in 0.2M PBS (pH 6.0) to 5.335 U / mL. The coniferyl alcohol and docetaxel solutions were stored in the dark at 4°C, and the laccase solution was stored at -20°C, and dissolved on ice before use. The reaction was carried out at 25° C. under the condition of protecting from light, and the reaction time was 1 h. Immediately after the reaction, 100 μL of methanol was added to terminate the enzyme reaction, and placed on ice, protected from light, until sample in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com