2-Halo-5-ynyl-pyridylnicotinyl ligands
A representative, alkyl technology, applied in the field of regulating mammalian nicotinic acetylcholine receptors, can solve problems such as adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0182] Pharmacological properties. We measured the in vitro binding affinity of the ligands for defined receptor subtypes (α2β2, α2β4, α3β2, α3β4, α4β2, α4β4, α6β2, α6β4, and α7) expressed in stably transfected cell lines. work together. [ 3 H] echinobaphyllin ([ 3 H]EB) binds with high affinity to the agonist recognition sites of all defined receptor subtypes. Rat forebrain homogenates were included to allow comparison between heterologous and native α4β2 and α7 nAChRs. Regarding the binding affinity values (K i ), see Table 1.
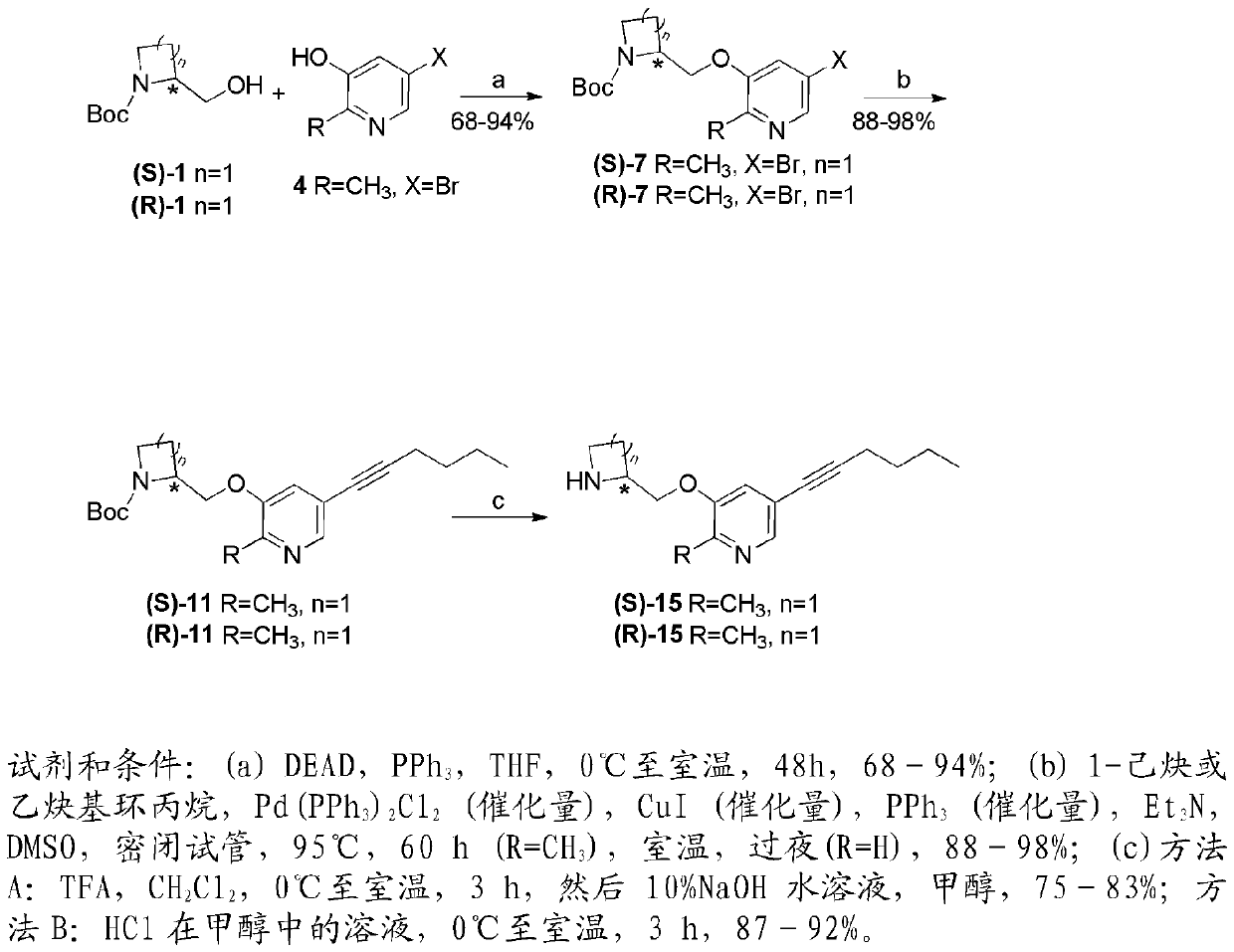
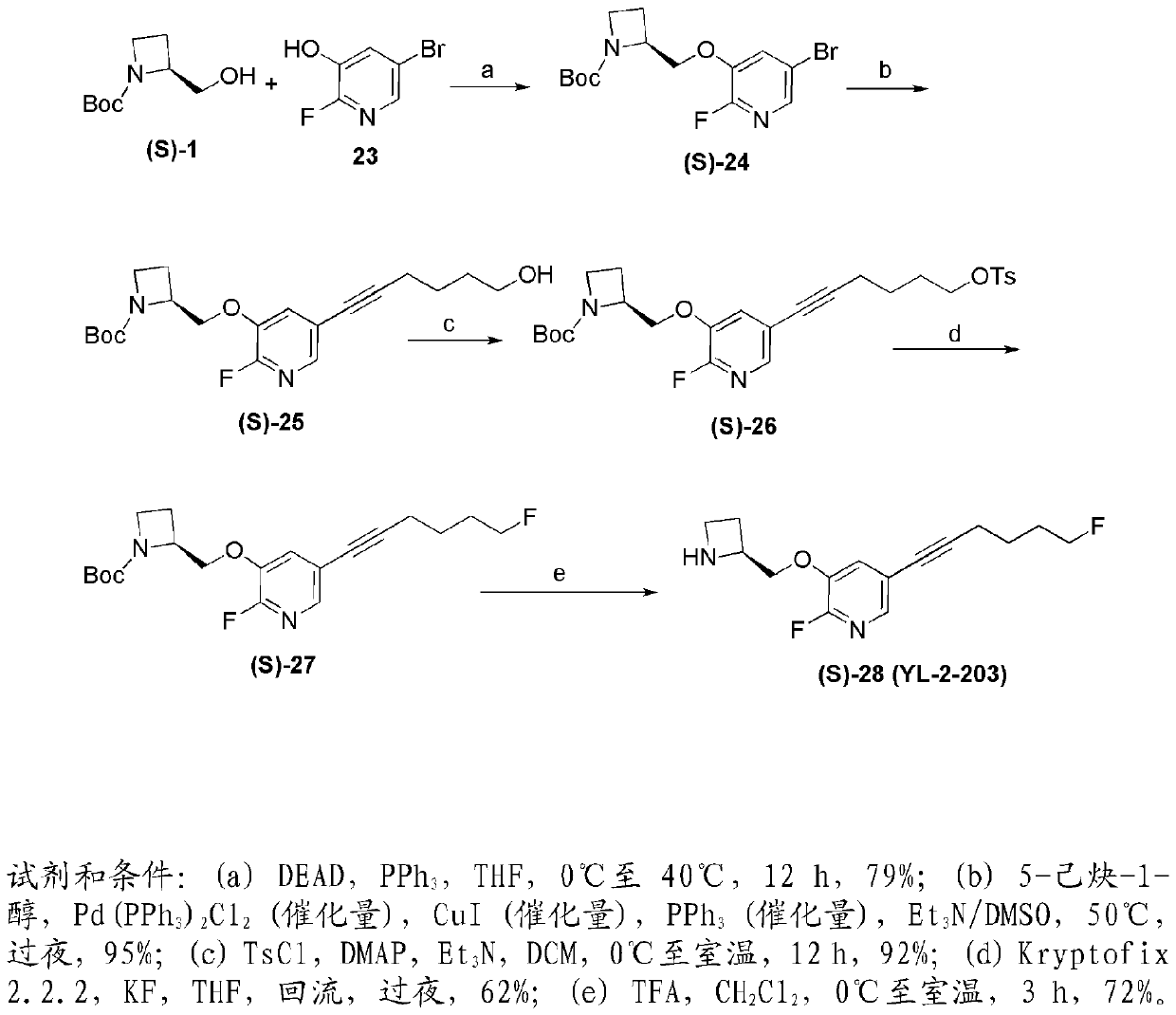
[0183] Compounds YL-1-127 and YL-2-203 are hereinafter abbreviated as (S)-15 and (S)-28, respectively. Compound (S)-15 represents an important benchmark compound that we have recently prepared, while (S)-28 is among the many compounds that make up the present invention. It was surprisingly found that the presence of a halogen at the 2-position of the pyridine ring, such as in (S)-28, greatly improves the desired binding activity and selectivi...
Embodiment 2
[0187] In vitro binding affinities of nAChR subtypes. 3 Binding affinities of novel 2-halo-5-substituted pyridyl analogues to defined rat nAChR subtypes as well as to the native nAChR of the rat forebrain were examined in a binding competition study of H]-ebapin . For comparison, the binding affinities of (-)-nicotine, varenicline and Sazetidine-A were obtained from parallel binding experiments. To determine the selectivity of these compounds for the 3 dominant nAChR subtypes α3β4, α4β2 and α7 in binding assays, K i Ratio of values (α3β4 / α4β2 and α7 / α4β2).
[0188] In cells expressing α3β4 and α4β2 nAChR subtypes by 86 Rb + Efflux assays to determine the functional properties of the ligands. The functional activity of each ligand was measured for its agonistic, antagonistic and desensitizing abilities. Agonist activity of each ligand was tested at 8 different concentrations. Responses were compared to those stimulated with 100 µM (-)-nicotine (near maximal effective c...
Embodiment 3
[0195] Design and Synthesis of Compounds. Compounds of the invention can be prepared by any conventional method that can be used for the preparation of similar compounds and is described in the Examples below.
[0196] The starting materials for the methods described in this patent application are known, or can be prepared by known methods from commercially available materials.
[0197] A compound of the invention can be converted into another compound of the invention using conventional methods.
[0198] The products of the reactions described herein are isolated by conventional means such as extraction, crystallization, distillation, chromatography, and the like.
[0199] Examples of nicotinic ACh receptor ligands of the invention can be prepared by the general methods described hereinafter. The general sequence of chemical reactions used to prepare the compounds described herein is shown in figure 1 and 2 middle.
[0200] General Chemical Methods. Unless otherwise indic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com