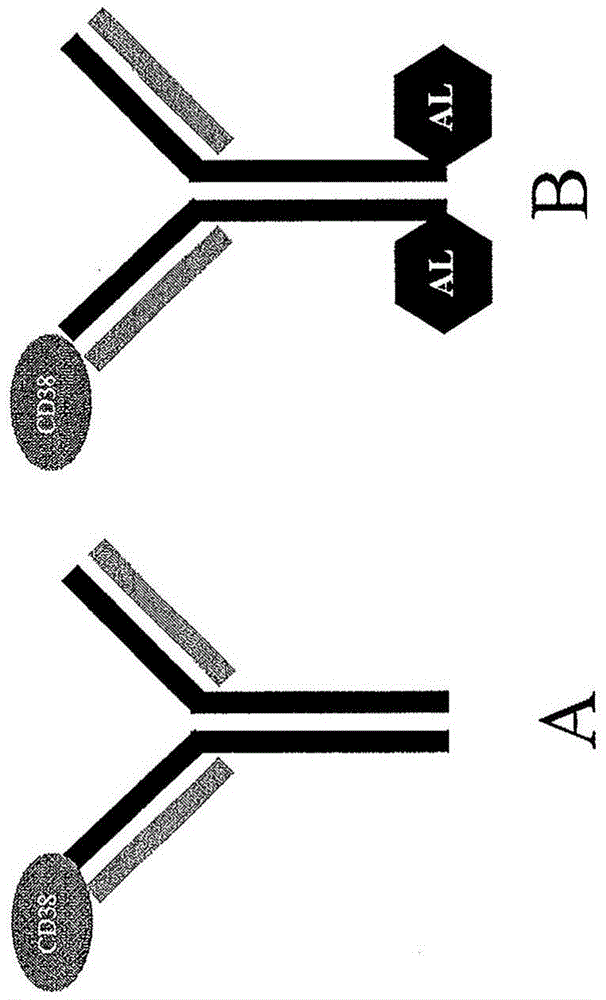
Anti-cd38 antibodies and fusions to attenuated interferon alpha-2b
A CD38-, antibody technology, applied in the direction of interferon, cytokines/lymphokines/interferons, antibodies, etc., can solve the problems of prolonging the half-life of IFN-α and not completely solving the problem of healthy cell activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
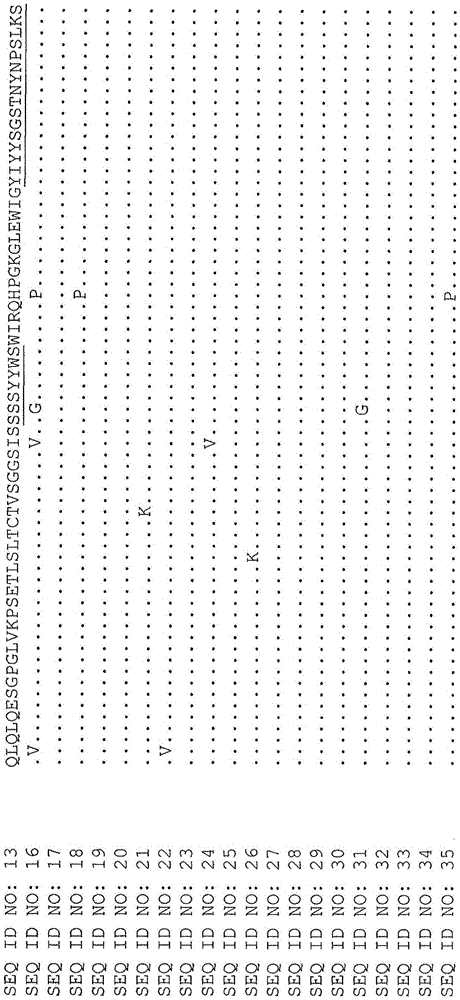
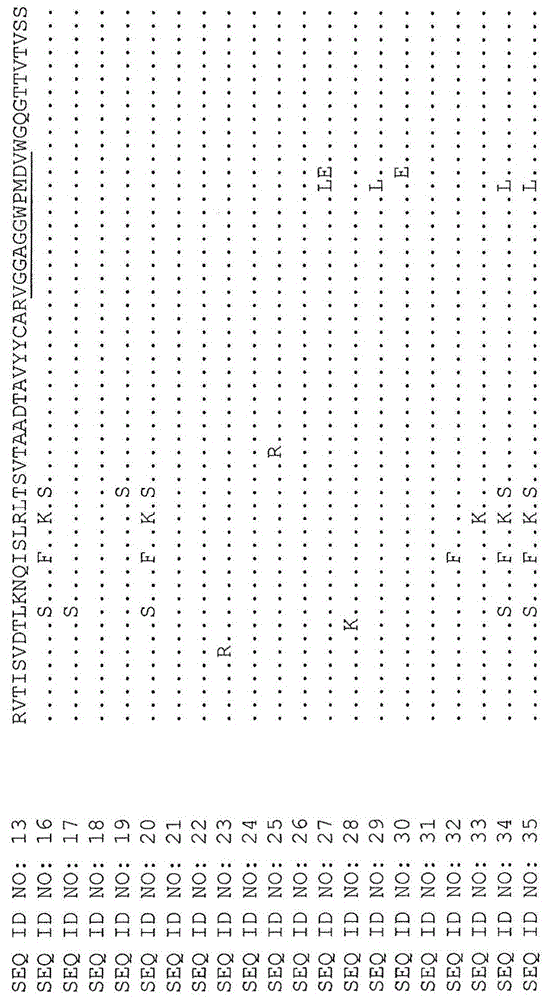
[0153] Optimized X355 / 02-HC-L0-IFN-α(A145D) IgG4
[0154] Other anti-CD38-attenuated IFN fusion proteins are described in PCT Application No. PCT / AU2012 / 001323. These include the antibody construct designated X355 / 02-HC-L0-IFN-alpha (A145D) IgG4 in the PCT application. In this specification, X355 / 02-HC-L0-IFN-α(A145D) IgG4 has been renamed as A02.1. The heavy chain sequence of the antibody comprises the amino acid sequence of SEQ ID NO:11, and the light chain sequence comprises the amino acid sequence of SEQ ID NO:12. The variable light chain (SEQ ID NO: 14) of A02.1 was co-expressed with its variable heavy chain (SEQ ID NO: 13) of A02.1 which contained the substitution S228P (EU numbering) (SEQ ID NO: 3) was formatted on the human IgG4 constant region. This antibody is referred to herein as X02.1. A02.1 includes a fusion to IFN-α2b, while X02.1 does not, although both antibodies have identical heavy and light chain sequences.
[0155] A BLAST study against a database of ...
example 2
[0213]In silico immunogenicity analysis of the A02.1 light chain amino acid sequence
[0214] Putative immunogenic epitopes were identified in the light chain variable region amino acid sequence of A02.1 using Epibase analysis software (Lonza, UK). To remove putative immunogenic epitopes, substitutions were introduced into the A02.1 variable light chain (Figure 4). The light chain with lower predicted immunogenicity was co-expressed in HEK293E cells with the A02.12 heavy chain variable region (SEQ ID NO:34) fused to A145D - Attenuated IFN formatted on IgG4 constant region containing substitution S228P. The antibody variants produced are detailed in FIG. 10 .
[0215] Table 10
[0216]
[0217]
[0218]
[0219] The above antibodies were analyzed for protein expression, CD38 binding by SPR, and potency using cell culture supernatant screen, as described in Example 5. The results of these experiments are detailed in Table 11. These data indicate that some residue s...
Embodiment 3
[0225] Multiple amino acid substitutions resulted in optimized A02.1 variants
[0226] Highly Optimized Anti-CD38 Antibody and Anti-CD38 Attenuated IFN Fusion Protein by Combining Substitutions Improving Immunogenicity, Manufacturability or Potency of Anti-CD38 Antibodies As Described Above Into a Single Gene Structure given. Table 12 summarizes the substitutions of such combinations and details the heavy and light chain combinations that were co-expressed in HEK293E cells and subsequently tested.
[0227] Table 12
[0228]
[0229]
[0230]
[0231]
[0232] Each antibody described in Table 12 was analyzed for protein expression, CD38 binding by SPR and titers using cell culture supernatants. The generated data are provided in Table 13. These results demonstrate that in silico predicted beneficial combinatorial substitutions resulted in several anti-CD38 attenuated IFN fusion proteins expressed and functionally potent in annexin V, caspase and cell proliferatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com