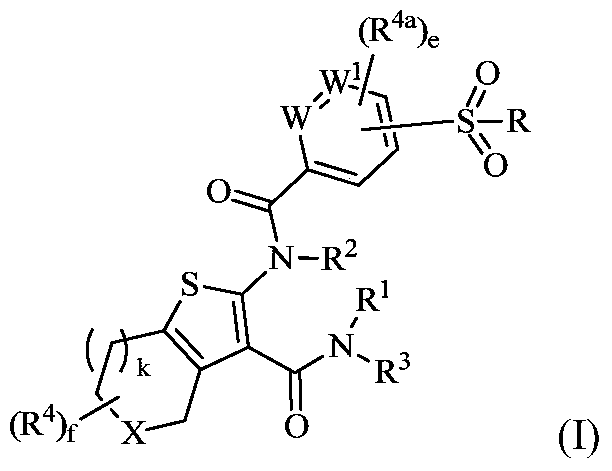
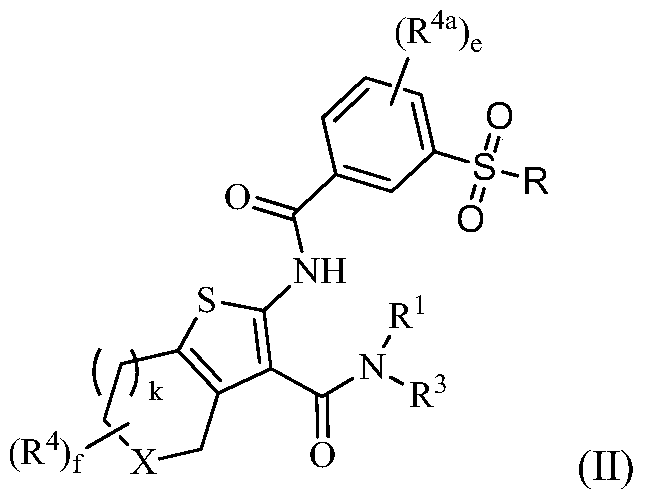
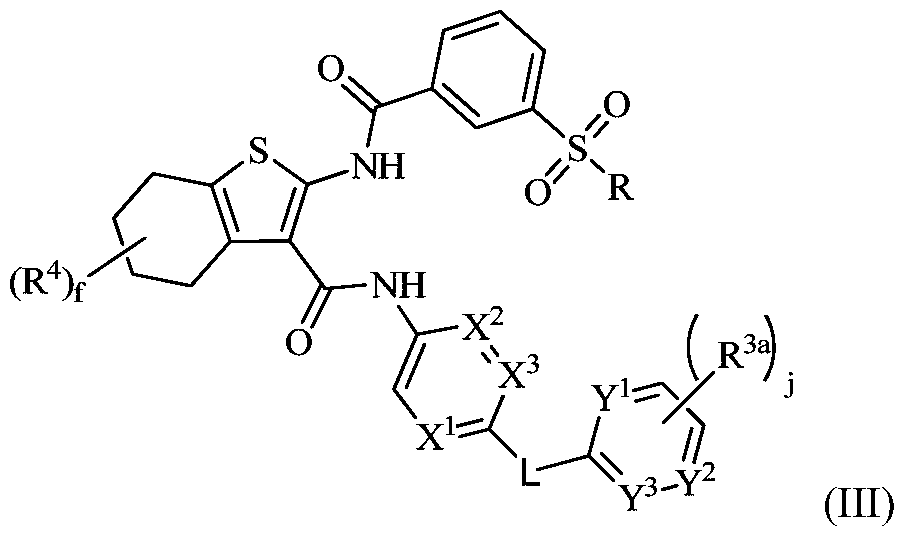
Tetrahydrobenzothiophene compound
A compound and alkyl technology, applied in the field of medicine, can solve problems such as poor drug compliance and increased serum calcium concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0308] 1-((3-((3-((4-(4-Carboxyphenethyl)phenyl)carbamoyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl )carbamoyl)phenyl)sulfonyl)indoline-5-carboxylic acid
[0309]
[0310] Step 1. 1-((3-((3-((4-(4-(methoxycarbonyl)phenethyl)phenyl)carbamoyl)-4,5,6,7-tetrahydrobenzo[ b] Methyl thiophen-2-yl)carbamoyl)phenyl)sulfonyl)indoline-5-carboxylate
[0311] Methyl indoline-5-carboxylate (177 mg, 1.0 mmol) was dissolved in pyridine (4 mL), 4-(4-(2-(3-(chlorosulfonyl)benzamido)-4 was added , 5,6,7-tetrahydrobenzo[b]thiophene-3-carboxamido)phenethyl)benzoic acid methyl ester (800 mg, 1.26 mmol) was heated to reflux for 18 hours. The reaction solution was diluted with ethyl acetate (80 mL) and washed with 0.1 mol / L hydrochloric acid (10 mL×3). The organic phase was dried over anhydrous sodium sulfate and concentrated. The crude product was purified by silica gel column chromatography (petroleum ether / dichloromethane (v / v)=1 / 1) to obtain 491 mg of yellow solid, yield: 63.2%.
[0312] M...
Embodiment 2
[0318] 1-((3-((3-((4-(4-Carboxyphenethyl)phenyl)carboxamide)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl) Formamide)phenyl)sulfonyl)decahydroquinoline-6-carboxylic acid
[0319]
[0320] Step 1. Methyl decahydroquinoline-6-carboxylate
[0321] Quinoline-6-carboxylate tert-methyl ester (2.08g, 11.1mmol) was added to glacial acetic acid (20mL), then platinum dioxide (0.40g, 1.7mmol) was added, and the mixture was reacted at 70°C for 17 hours under a hydrogen atmosphere . Cooled to room temperature, filtered with suction, washed with methanol (15 mL), the filtrate was spin-dried, the residue was dissolved in water (40 mL), adjusted to pH=10 with saturated sodium carbonate, extracted with ethyl acetate (80 mL×3), the organic phase was washed with anhydrous sulfuric acid Dry over sodium, filter, and concentrate the filtrate under reduced pressure. The residue was purified by silica gel column chromatography (dichloromethane / methanol (v / v)=30 / 1) to obtain 1.60 g of a brown oil, yi...
Embodiment 3
[0331] 4-(4-(2-(3-((1-Acetylhexahydro-1H-pyrrolo[3,4-b]pyridin-6(2H)-yl)sulfonyl)benzamido)-4 ,5,6,7-Tetrahydrobenzo[b]thiophene-3-carboxamido)phenethyl)benzoic acid
[0332]
[0333] Step 1. 6-((3-((3-((4-(4-(methoxycarbonyl)phenethyl)phenyl)carbamoyl)-4,5,6,7-tetrahydrobenzo[ b]Thien-2-yl)carbamoyl)phenyl)sulfonyl)octahydro-1H-pyrrolo[3,4-b]pyridine-1-carboxylic acid tert-butyl ester
[0334] Octahydro-1H-pyrrolo[3,4-b]pyridine-1-carboxylic acid tert-butyl ester (355 mg, 1.57 mmol) and triethylamine (0.44 mL, 3.2 mmol) were dissolved in dichloromethane (20 ml), Cool to -25°C. Then 4-(4-(2-(3-(chlorosulfonyl)benzamido)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxamido)phenethyl was added dropwise yl)methyl benzoate (1.00 g, 1.57 mmol) in dichloromethane (15 mL) was added over 15 minutes. Stir at -25°C for 5 minutes, then move to room temperature and stir for 18 hours. A saturated solution of ammonium chloride (20 mL) was added, the aqueous phase was extracted with dic...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap