Piribedil hydrophilic gel membrane controlled and sustained release reparation and preparation method thereof
A technology of hydrophilic gel and sustained-release preparation, applied in the field of medicine, can solve the problems of poor effect, decreased drug efficacy, poor stability, etc., and achieve the effects of improving compliance, improving drug efficacy and stable release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
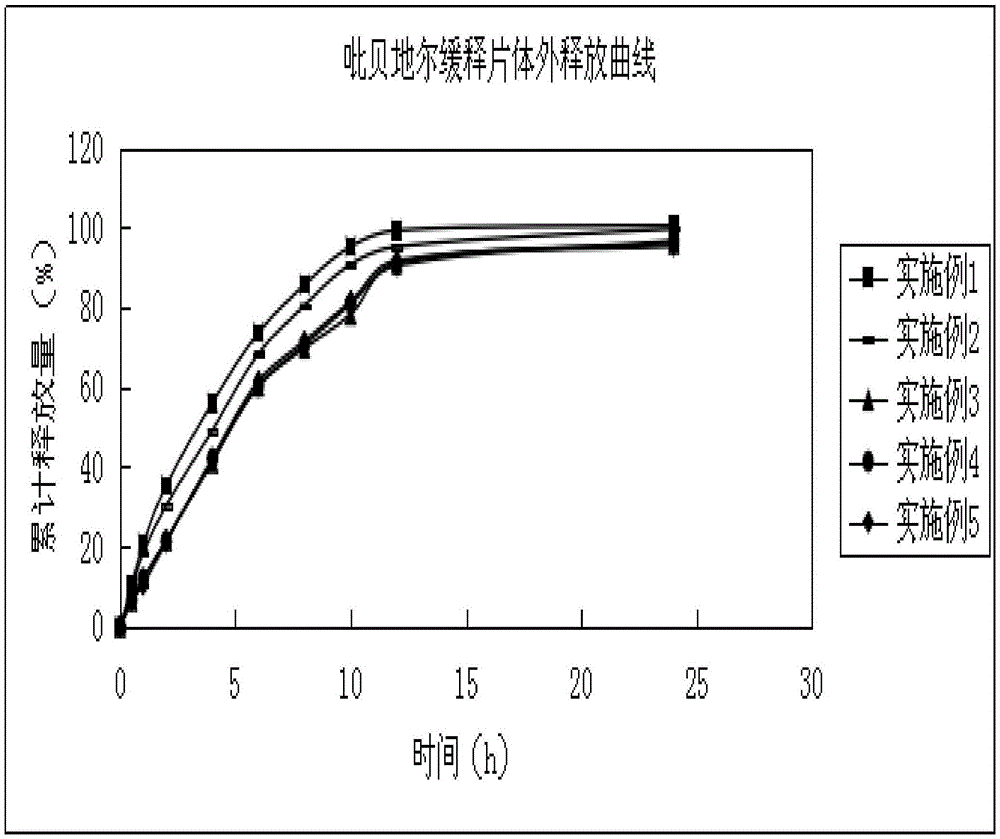
Image
Examples
Embodiment 1
[0038] (1) Prescription:
[0039]
[0040]
[0041] (2) Preparation method:
[0042] (1) Micronizing the piribedil so that the particle size is 10-50 μm;
[0043] (2) each auxiliary material is crossed 80 mesh sieves, for subsequent use;
[0044] (3) Weigh the prescribed amount of piribedil, divide the filling agent into 20 equal parts, add and mix in turn, and shake at a frequency of 10 times / s for 10 minutes;
[0045] (4) Add an appropriate amount of adhesive to make a soft material, pass through a 26-mesh sieve to granulate, and dry in a 60-degree oven for 1 hour;
[0046] (5) Pass through a 26-mesh sieve for granulation, add skeleton materials and mix evenly; add an appropriate amount of lubricant, mix evenly, and compress into tablets to obtain tablet cores.
[0047] (6) Dissolve the slow-release coating material in ethanol, stir to dissolve completely, put the plain tablet in the coating pan, adjust the temperature (50-60°C) and rotation speed (5-15rpm), and inc...
Embodiment 2
[0051] (1) Prescription:
[0052]
[0053]
[0054]
[0055] (2) Preparation method:
[0056] (1) Micronizing the piribedil so that the particle size is 10-50 μm;
[0057] (2) each auxiliary material is crossed 80 mesh sieves, for subsequent use;
[0058] (3) Weigh the prescribed amount of piribedil, divide the filling agent into 20 equal parts, add and mix in turn, and shake at a frequency of 10 times / s for 10 minutes;
[0059] (4) Add an appropriate amount of adhesive to make a soft material, pass through a 26-mesh sieve to granulate, and dry in a 60-degree oven for 1 hour;
[0060] (5) Pass through a 26-mesh sieve for granulation, add skeleton materials and mix evenly; add an appropriate amount of lubricant, mix evenly, and compress into tablets to obtain tablet cores.
[0061] (6) Dissolve the slow-release coating material in ethanol, stir to dissolve completely, put the plain tablet in the coating pan, adjust the temperature (50-60°C) and rotation speed (5-15rpm...
Embodiment 3
[0066] (1) Prescription:
[0067]
[0068]
[0069] (2) Preparation method:
[0070] (1) Micronizing the piribedil so that the particle size is 10-50 μm;
[0071] (2) each auxiliary material is crossed 80 mesh sieves, for subsequent use;
[0072] (3) Weigh the prescribed amount of piribedil, divide the filling agent into 20 equal parts, add and mix in turn, and shake at a frequency of 10 times / s for 10 minutes;
[0073] (4) Add an appropriate amount of adhesive to make a soft material, pass through a 26-mesh sieve to granulate, and dry in a 60-degree oven for 1 hour;
[0074] (5) Pass through a 26-mesh sieve for granulation, add skeleton materials and mix evenly; add an appropriate amount of lubricant, mix evenly, and compress into tablets to obtain tablet cores.
[0075] (6) Dissolve the slow-release coating material in ethanol, stir to dissolve completely, put the plain tablet in the coating pan, adjust the temperature (50-60°C) and rotation speed (5-15rpm), and inc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com