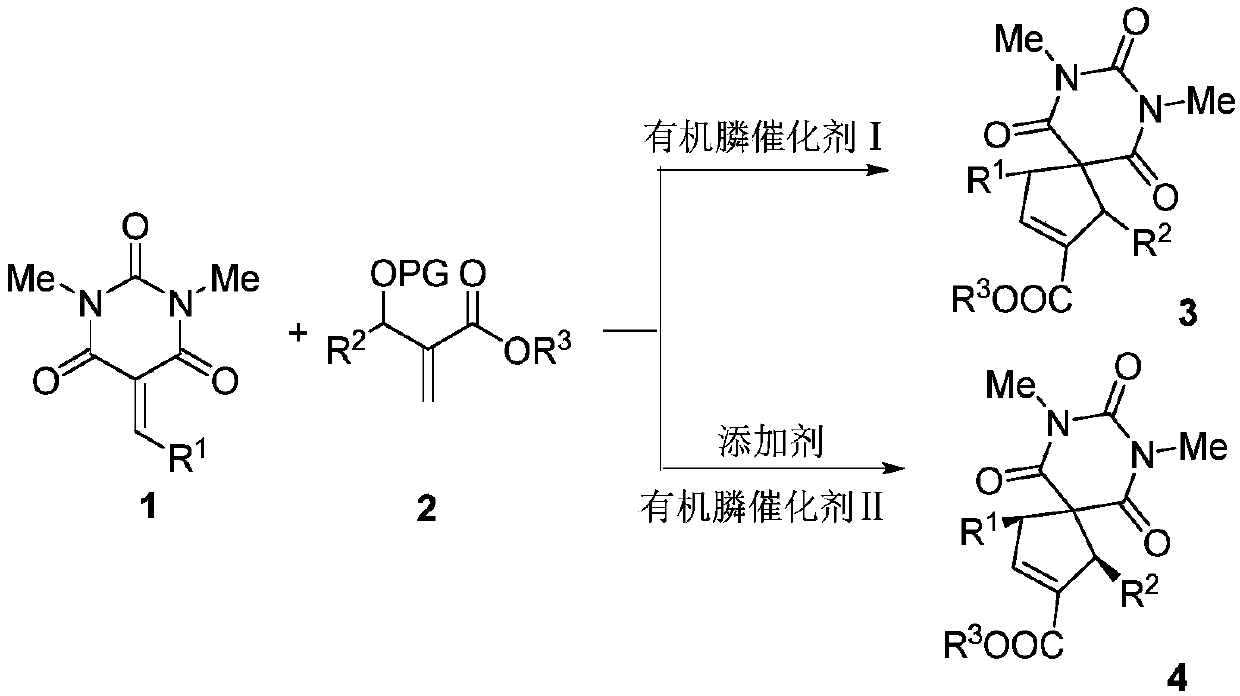
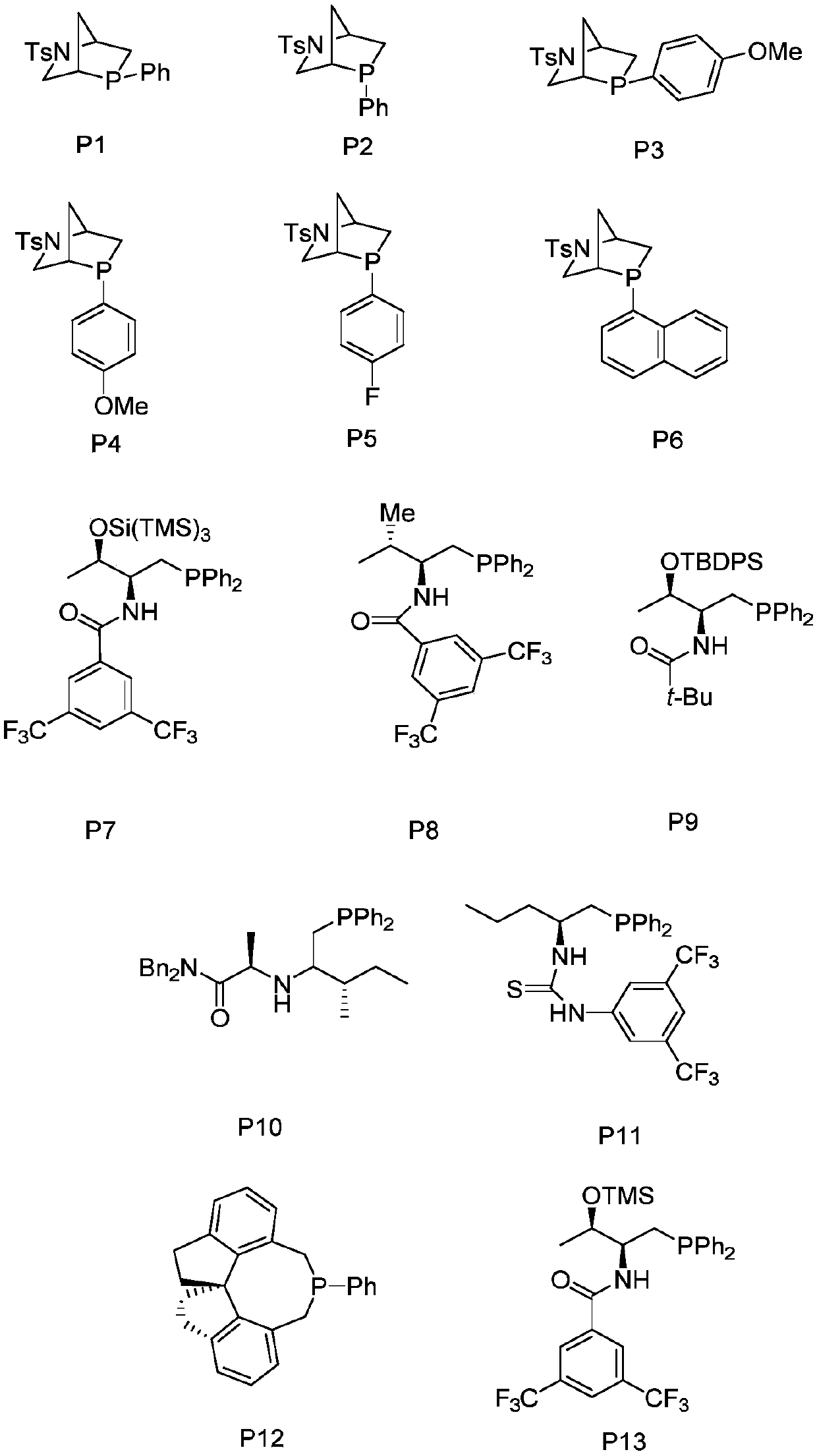
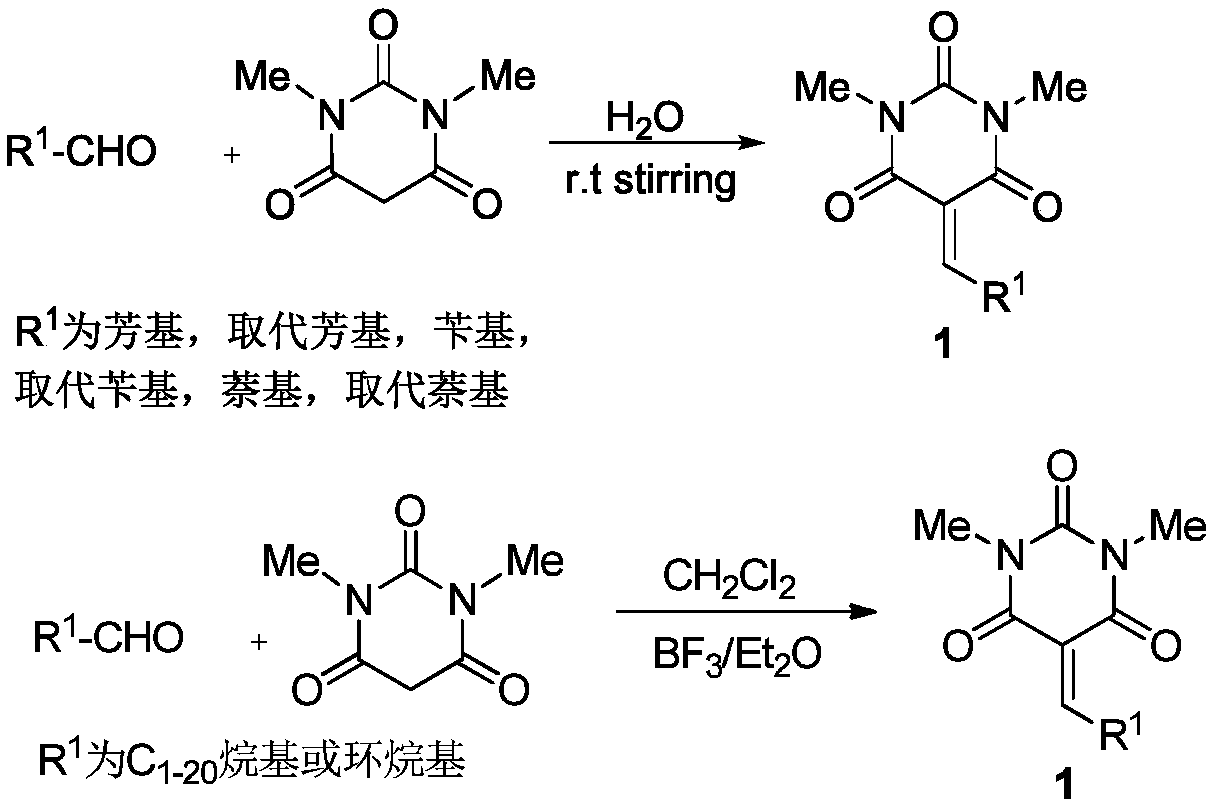
Barbituric acid-cyclopentene spiro compound and preparation method thereof
A technology of spiro compound and barbiturate is applied in the field of organic compound synthesis to achieve high stereoselectivity and enantioselectivity, increase diversity and expand applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032]Put 0.0244g (0.100mmol) of compound 1a, 0.0350g (0.120mmol) of compound 2a and 3mL of 1,2-dichloroethane into a dry 15mL Shrek tube, add 0.020mmol of methyl diphenylphosphine and mix well Carry out a cycloaddition reaction. In the reaction system, the molar ratio of compound 1a to compound 2a is 1:1.2, methyldiphenylphosphine accounts for 20% of the molar percentage of compound 1a, stirred at 40°C for 24 hours, and concentrated through the column with a rotary evaporator (Ethyl acetate:petroleum ether=1:10, v / v), 23.4 mg of product 3aa was obtained, with a yield of 56%.
Embodiment 2
[0034]
[0035] Put 0.0244g (0.100mmol) of compound 1a, 0.0350g (0.120mmol) of compound 2a and 3mL of toluene into a dry 15mL Shrek tube, add 0.020mmol of methyl diphenylphosphine and mix well for cycloaddition reaction. In the reaction system, the molar ratio of compound 1a to compound 2a is 1:1.2, methyldiphenylphosphine accounts for 20% of the molar percentage of compound 1a, stirred at 40°C for 24 hours, and concentrated through the column with a rotary evaporator (Ethyl acetate:petroleum ether=1:10, v / v), 36.8 mg of product 3aa was obtained, with a yield of 89%.
[0036] It can be seen that the effect is very excellent when selecting toluene as the solvent.
Embodiment 3
[0038]
[0039] Put 0.0244g (0.100mmol) of compound 1a, 0.0350g (0.120mmol) of compound 2a and 3mL of toluene into a dry 15mL Shrek tube, add 0.020mmol of dimethylphenylphosphine and mix well for cycloaddition reaction. In this reaction system, the molar ratio of compound 1a to compound 2a is 1:1.2, dimethylphenylphosphine accounts for 20% of the molar percentage of compound 1a, stirred at 40°C for 24 hours, concentrated through the column with a rotary evaporator (Ethyl acetate:petroleum ether=1:10, v / v), 31.5 mg of product 3aa was obtained, with a yield of 76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com