2,3-Disubstituted indoline derivatives and preparation method thereof
An indoline, disubstituted technology, applied in the field of 2,3-disubstituted indoline derivatives and their preparation, can solve the problems of expensive reagents, harsh reaction conditions and the like, and achieve the effect of increasing diversity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
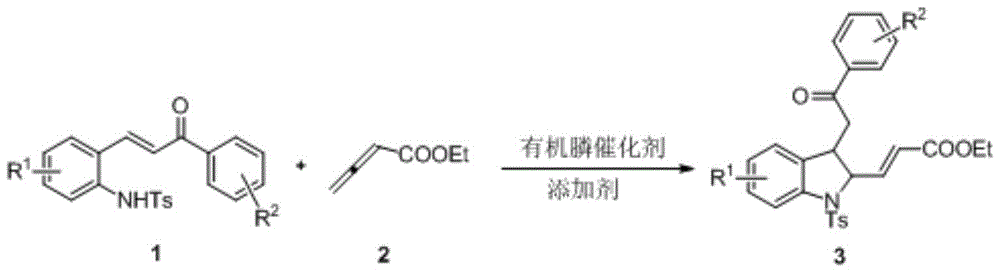
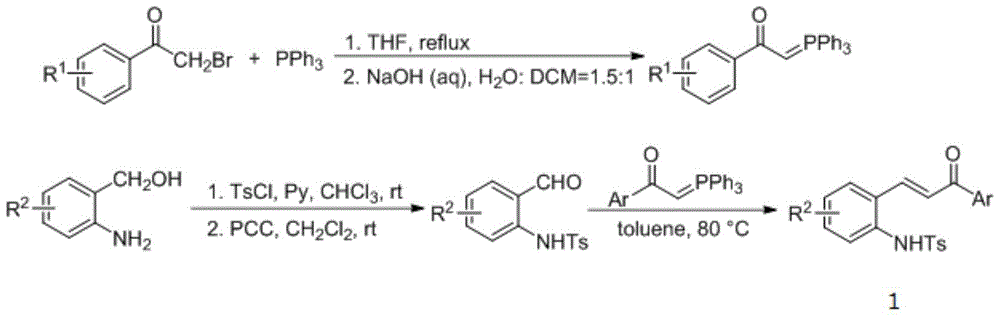
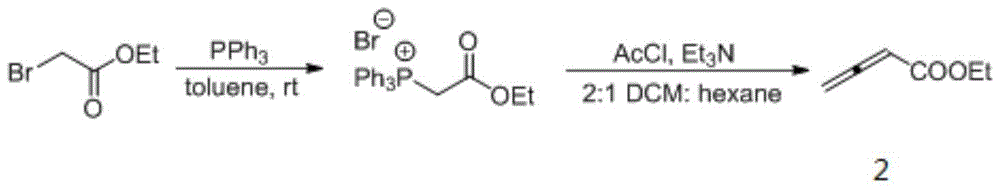
[0024] Put 0.0754g (0.200mmol) of compound 1a, 3mL of dichloromethane and 21ul (0.300mmol) of ethyl butadienoate 2 into a dry 15mL Shrek tube, add 10.5mg (0.040mmol) of triphenylphosphine, acidic Add benzoic acid and mix well for cycloaddition reaction. In this reaction system, the molar ratio of compounds 1a and 2 is 1:1.5, and the molar percentage of compound 1a is 100%. Stir at 25° C. for 48 hours, and use a rotary evaporator to concentrate the reaction solution and pass it through a column (ethyl acetate: ethyl acetate: Petroleum ether=1:5, v / v) to obtain 7.9 mg of 2,3-disubstituted indoline 3a, yield 8%. From the NMR data listed below, the structure of the obtained product is correct.
[0025] NMR data for 3a:
[0026] 1 H NMR (300MHz, CDCl 3 )δ7.8(d,J=8.1Hz,1H),7.8-7.7(m,3H),7.6-7.5(m,3H),7.4-7.3(m,2H),7.2-7.0(m,5H) ,6.3(dd,J=15.5,1.7Hz,1H),4.6(m,1H),4.2(q,J=7.1Hz,2H),3.4(m,1H),2.8(dd,J=18.7,4.0 Hz, 1H), 2.3 (s, 3H), 1.8 (dd, J=18.7, 10.5 Hz, 1H), 1.3 (...
Embodiment 2
[0029]
[0030] Put 0.0754g (0.200mmol) of compound 1a, 3mL of dichloromethane and 21ul (0.300mmol) of ethyl butadienoate 2 into a dry 15mL Shrek tube, add 40ul (0.040mmol) of trimethylphosphine (1M of tetrahydrofuran solution), acid additive benzoic acid, and mix well for cycloaddition reaction. In this reaction system, the molar ratio of compounds 1a and 2 is 1:2.5, and the molar percentage of compound 1a is 100%, stirring at 25° C. for 48 hours, and concentrating the reaction solution with a rotary evaporator and passing through a column (ethyl acetate: ethyl acetate: Petroleum ether=1:5, v / v) to obtain 34.2 mg of 2,3-disubstituted indoline 3a, yield 35%.
Embodiment 3
[0032]
[0033] Put 0.0754g (0.200mmol) compound 1a, 3mL tetrahydrofuran and 21ul (0.300mmol) ethyl butadienoate 2 into a dry 15mL Shrek tube, add 17ul (0.060mmol) tributylphosphine, 4.88mg (0.040 mmol) acid additive benzoic acid, mix well to carry out cycloaddition reaction. In this reaction system, the molar ratio of compounds 1a and 2 is 1:2, and the molar percentage of compound 1a is 100%. Stir at 40° C. for 24 hours, and use a rotary evaporator to concentrate the reaction solution and pass it through a column (ethyl acetate: ethyl acetate: Petroleum ether=1:5, v / v) to obtain 69.4 mg of 2,3-disubstituted indoline 3a with a yield of 71%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com