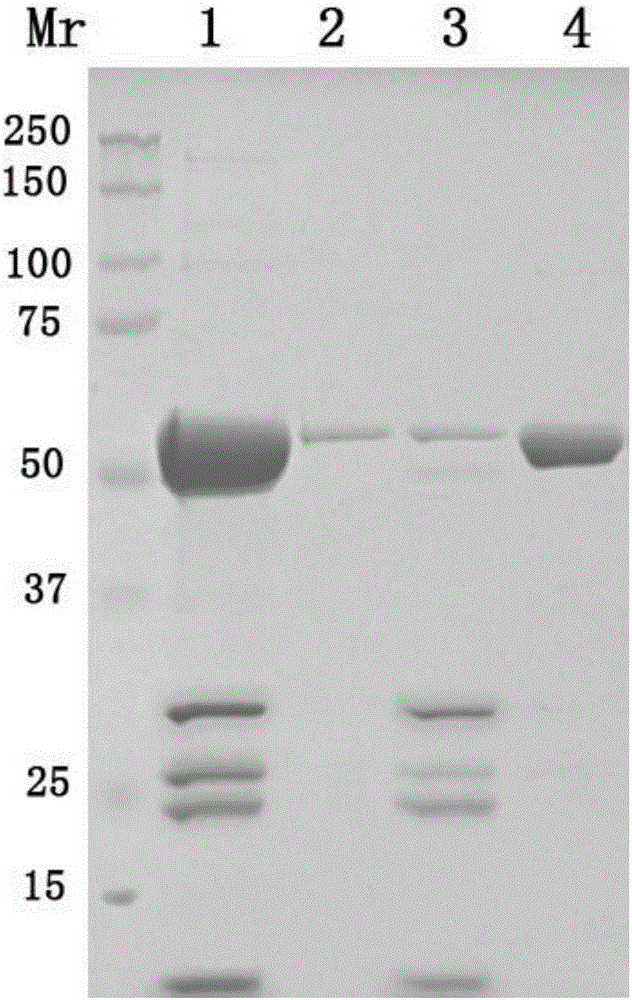
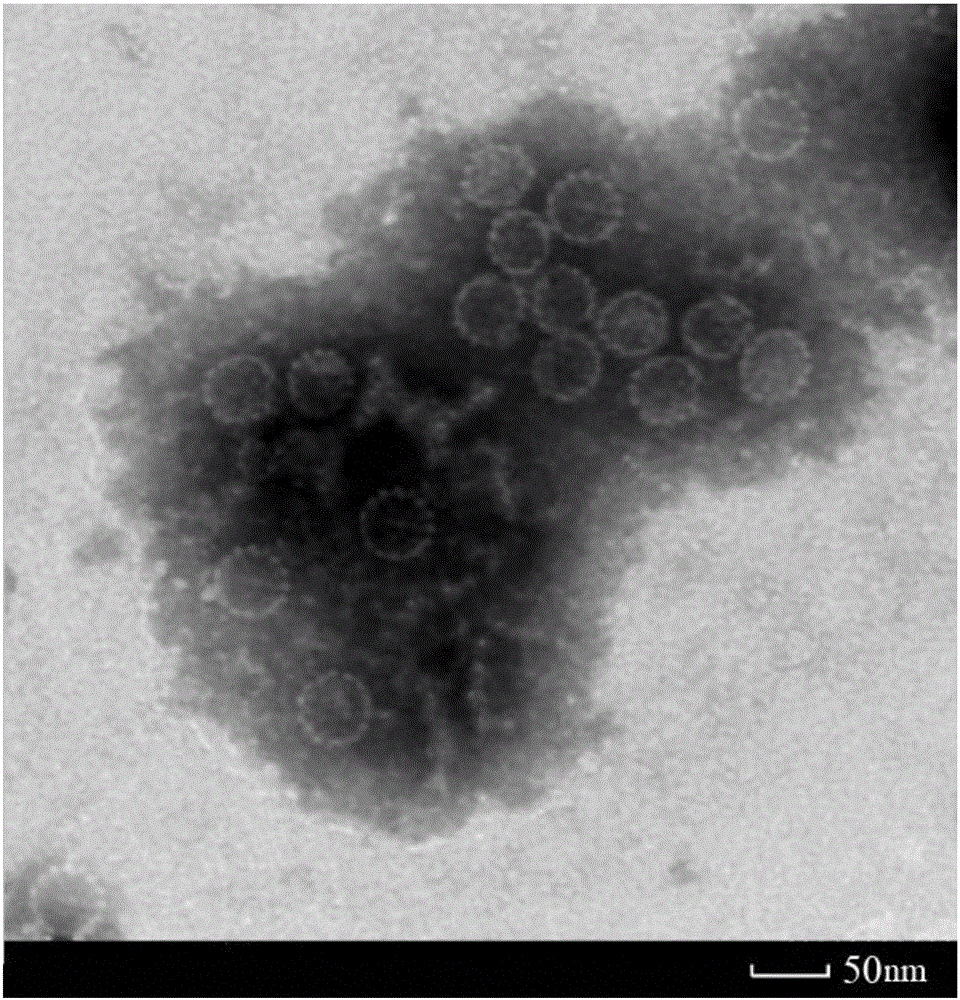
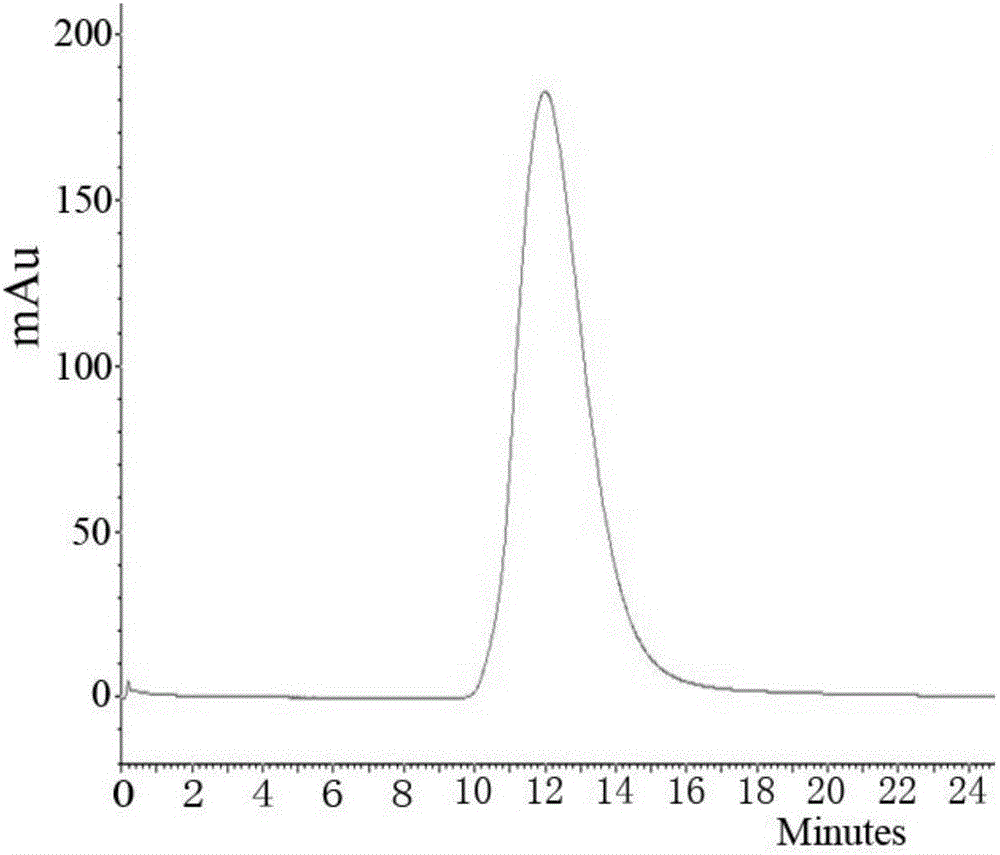
Method for preparing human papilloma virus (HPV) L1 protein virus-like particles (VLP)
A technology of human papillomavirus and L1 protein, which is applied in the field of virus-like particles, can solve the problems of insufficient uniformity of VLP, poor uniformity of virus-like particles, and lower recovery rate of target proteins, etc., to achieve easy industrial production and long service life , cost-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1) Preliminary purification
[0036] Bacterial cell disruption: Harvest the Pichia pastoris cells transformed by fermentation and culture and express HPV16L1VLP, wash the bacterial cells three times with physiological saline, remove the remaining components of the fermentation medium, and freeze the collected bacterial cells at -20°C. Take out the frozen bacterial cells, weigh them and add 0.2M NaCl, 5mM EDTA-2Na, 0.05% Tween-80, pH 8.050mM PB buffer solution pre-cooled to 0°C at a ratio of 1:10 to the bacterial liquid, stir and thaw Until it is completely melted, high-pressure homogenate is broken, and the operating pressure of 1200bar is circulated twice to break more than 95% of the bacterial cells. The lysate is stirred at 4°C for 1 hour to completely dissolve the target protein in the lysed suspension. At the same time, 20mM DTT was added to completely depolymerize the target protein in a less uniform form into a more uniform L1 protein pentamer.
[0037] Centrifu...
Embodiment 2
[0045] 1) Preliminary purification
[0046] Sample pretreatment: Harvest the Escherichia coli cells transformed by fermentation and culture and express HPV18L1VLP, wash the bacterial cells once with physiological saline to remove the remaining components of the fermentation medium, and freeze the collected bacterial cells at -20°C. Take out the frozen bacterial cells, weigh them and add 0.2M NaCl, 5mM EDTA-2Na, 0.05% Tween-80, pH 8.050mM PB buffer solution pre-cooled to 0°C at a ratio of 1:10 to the bacterial liquid, stir and thaw Until it is completely melted, high-pressure homogenate is broken, and the operating pressure of 700bar is circulated twice, which can break more than 95% of the bacterial cells. The lysate is stirred at 4°C for 1 hour to completely dissolve the target protein in the lysed suspension. At the same time, 20mM DTT was added to completely depolymerize the target protein in a less uniform form into a more uniform L1 protein pentamer. Centrifuge the lysed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap