Gene cloning, expression and application of recombinant human fibroblast growth factor-20
A FGF-20, prokaryotic expression technology, applied in the direction of medical preparations containing active ingredients, recombinant DNA technology, microorganism-based methods, etc., to achieve the effects of reducing production costs, increasing yield and expression, and simplifying purification steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1F
[0031] The construction of embodiment 1 FGF-20 protein high-efficiency expression engineering bacterium
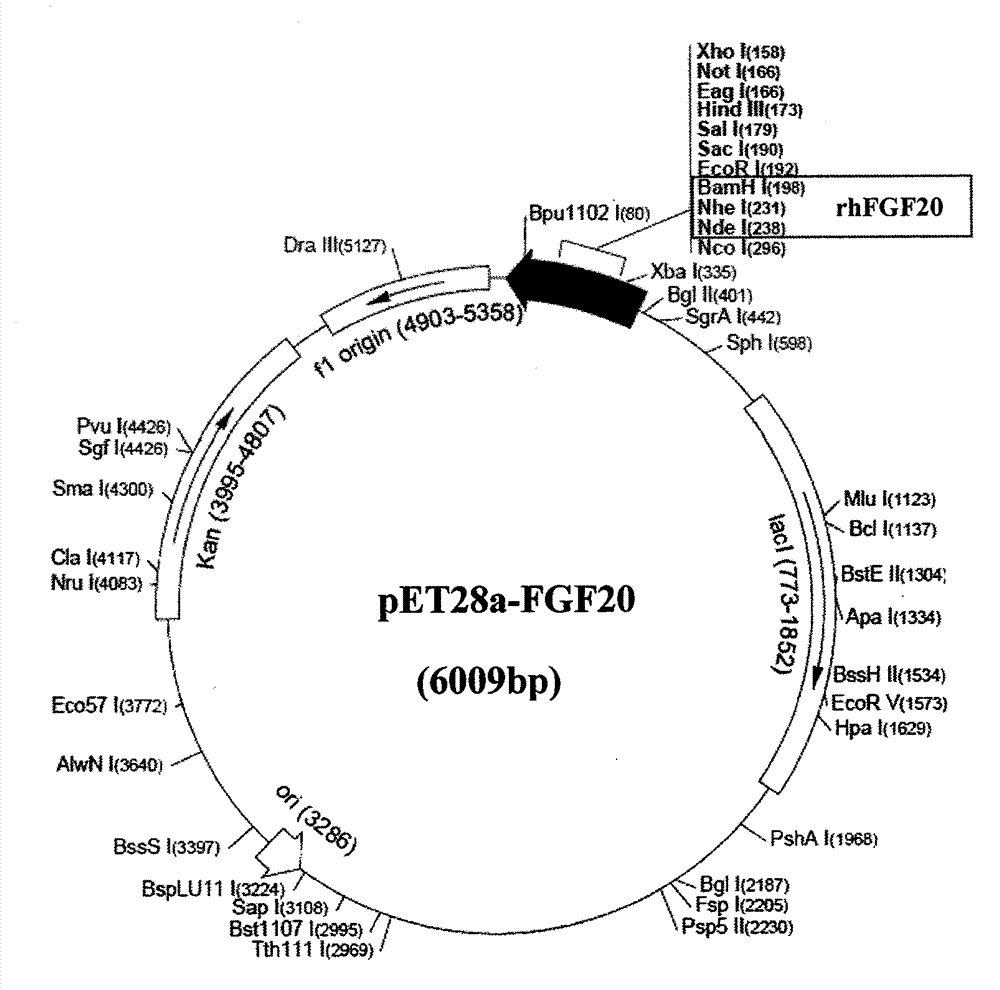
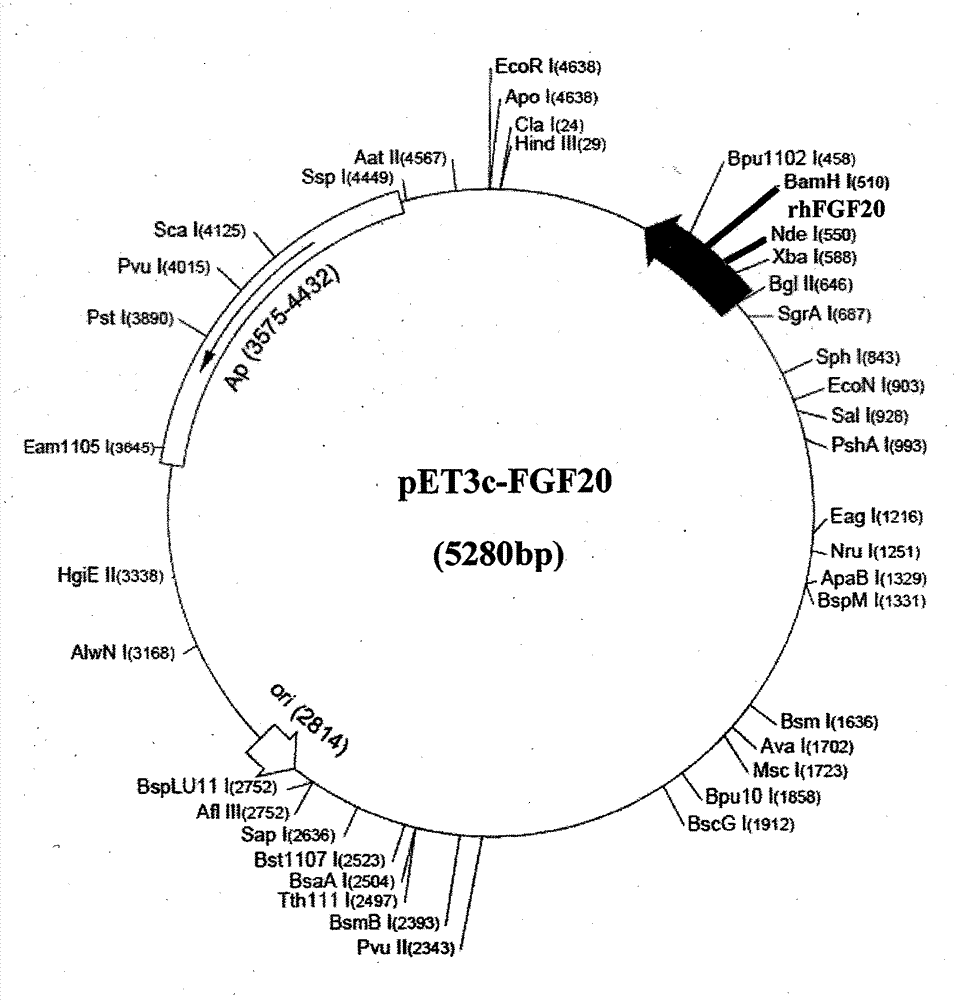
[0032] According to the human FGF-20 natural sequence (accession number: BC098128.1) and amino acid sequence published in GenBank, optimize according to the codon preference of Escherichia coli, and design the coding sequence primers of rhFGF-20 without changing the amino acid sequence . The full-length nucleotide sequence of rhFGF-20 was obtained by PCR amplification. Add start codon and stop codon to the 5' end and 3' end of the preferred sequence of rhFGF-20, respectively, and introduce specific enzyme cutting sites Nde I and BamH I, and then treat rhFGF with NdeI and BamHI double enzymes respectively -20 gene and expression vector pET3a-c (or pET28a), digested at 37°C for 3h to 4h, recovered the corresponding fragments, ligated overnight at 16°C with T4 DNA ligase, and constructed a recombinant expression vector (see figure 1 , figure 2 ). The ligation product was...
Embodiment 2
[0033] The establishment of embodiment 2 FGF-20 protein high-density culture method
[0034] In the present invention, the fermentation conditions for expressing rhFGF-20 by engineered bacteria are not particularly limited. Conventional fermentation conditions in the art can be used. Usually, engineering bacteria are composed of tryptone, yeast powder, ammonium salt, etc. as nitrogen source, glucose, glycerin, etc. basal medium; and through the regulation of control parameters (pH, temperature, dissolved oxygen, stirring speed, etc.) , prolong the logarithmic growth cycle, and significantly increase the cell yield and expression level. Specifically: Inoculate the FGF-20 engineering strain into LB medium at a ratio of 1:200-300 for activation to prepare first-generation seeds, cultivate for 3h-4h, and wait for A 600 reach about 0.8-1.0, inoculate into LB medium containing phosphate buffered saline at a ratio of 1:10-20 to amplify and prepare second-generation seeds, cultivat...
Embodiment 3
[0037] The establishment of embodiment 3FGF-20 protein purification method
[0038] 1. Bacteria fragmentation
[0039] After the cells were thawed at room temperature, they were fully suspended in cell lysis buffer (20mM Tris-HCl, 2mM EDTA-2Na, 0.1M NaCl, Triton X-100, 0.2% deoxycholic acid at a ratio of 1:20 (g:mL) Sodium, pH7.5), high pressure homogenization. The crushing method is 200bar circulation once, and then 800bar homogenization for 1~2 times. After microscopic examination shows no intact E. coli in the visible range, centrifuge at 20,000rpm, 4°C, 30min, discard the supernatant, and collect the precipitate.
[0040] 2. Inclusion body cleaning
[0041] Take the above-mentioned precipitate after centrifugation, add 10 times the amount of washing buffer (20mM Tris-HCl, 2mM EDTA-2Na, 0.1M NaCl, Triton X-100, 0.2% sodium deoxycholate, pH7.5) to fully stir and wash, After 10min to 15min, centrifuge at 20000rpm at 4°C for 30min, discard the supernatant, and collect the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com