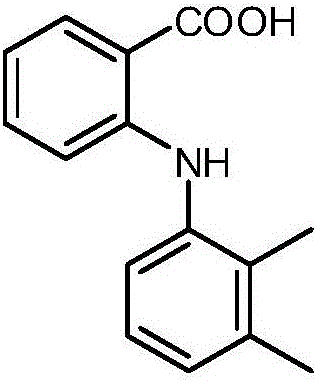
Synthesis method of mefenamic acid
A technology of mefenamic acid and its synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of low production cost, dark product color, low product yield, etc., and achieve improved yield , reduce the amount of tar produced, reduce the effect of side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 mefenamic acid
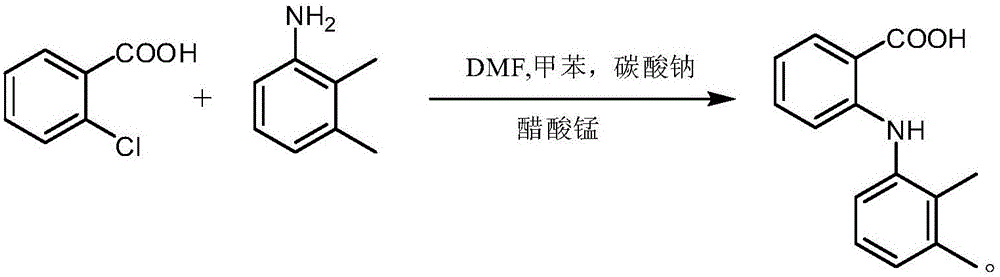
[0030] Put 10g of DMF into a 250mL four-neck flask, raise the temperature to 80°C, put in 27g of o-chlorobenzoic acid, until the o-chlorobenzoic acid is completely dissolved, add 25g of sodium carbonate to form a salt, and keep at 80°C for half an hour. Connect the water separator, add 25g of toluene to separate the water until no water comes out. Afterwards, 0.5 g of manganese acetate catalyst and 22.5 g of 2,3-dimethylaniline were added, and the temperature was kept at 120-130°C. Sampling HPLC central control, when o-chlorobenzoic acid <1%, add 100mL water, add dilute sulfuric acid to adjust the pH to 2, filter with suction, and dry to obtain about 39.5g off-white solid with a molar yield of 94.8%. The nuclear magnetic detection data and The reaction formulas are as follows.
[0031] 1 H NMR(DMSO):δ2.10(s,3H,-CH 3 ),2.28(s,3H,-CH 3 ),6.68~7.90(m,7H,Ar-H),9.46(s,-NH).
[0032]
Embodiment 2
[0033] The preparation of embodiment 2 mefenamic acid
[0034] Put 10g of DMF into a 250mL four-neck flask, raise the temperature to 80°C, put in 27g of o-chlorobenzoic acid, until the o-chlorobenzoic acid is completely dissolved, add 25g of sodium carbonate to form a salt, and keep at 80°C for half an hour. Connect the water separator, add 25g of toluene to separate the water until no water comes out. Afterwards, 0.5 g of catalyst manganese sulfate and 22.5 g of 2,3-dimethylaniline were added, and the temperature was kept at 120-130°C. Sampling is controlled by HPLC. When o-chlorobenzoic acid is <1%, add 100mL of water, add dilute sulfuric acid to adjust the pH to 2, filter with suction, and dry to obtain about 38.7g of off-white solid with a molar yield of 93.0%. The NMR detection data is as follows :
[0035] 1 H NMR(DMSO):δ2.10(s,3H,-CH 3 ),2.28(s,3H,-CH 3 ), 6.68~7.90 (m, 7H, Ar-H), 9.46 (s, -NH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com