Sequence capture method using specialized capture probes (heatseq)
A probe, sequence technology, applied in the field of sequence capture using specialized capture probes (HEATSEQ)
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: MIP probe library production and purification
[0062] exist figure 1 A protocol for converting MIP-precursors to MIPs is detailed in . figure 1 A shows an example for a MIP-precursor molecule. In this example, the MIP precursor was formed by synthesis on the MAS unit such that the precursor was formed on the array surface. The MIP precursor molecule in this example contains two 15 mer primer sites on the 5' and 3' ends. Near the end primer site there are two 20 mer sites X20 and Y20 that are target-specific regions that are complementary to a specific site that is a border for a specific target region in the sample. Between X20 and Y20 is a linker region, in this case a 30 mer sequence, which joins the two target-specific sequences together.
[0063] The MIP precursor was then amplified using two primers, in this case shown in figure 1 in B. There are both forward and reverse primers. The forward primer contains the same sequence as seen on the...
Embodiment 2
[0069] Example 2 : Use of the MIP probe library to capture regions of interest
[0070] The protocol from Example 1 above will generate a 70-mer MIP that can be used for hybridization to genomic DNA. For the purposes of these examples, this collection was named MIP480 mix. It is also readily recognized that such MIPs can be prepared for use with other forms of nucleic acid targets, including cDNA, RNA, etc. exist image 3 The hybridization and extension steps are depicted in , where MIP probes are contacted with genomic DNA.
[0071] In this example, approximately 750 ng of hgDNA or 2.25 x 105 hgDNA copies were used. Keeping the MIP:genome equivalent ratio at approximately 100:1, use 1 pg of each probe (500 pg = 0.5 ng of MIP480 mix). These MIP calculations assume that only 70 nucleotide MIP fragments are present. For hybridization reactions, use the following reagents:
[0072] Reagent volume 263 ng / µl Genomic DNA (Female, Promega) 3 µl 790 ng...
Embodiment 3
[0083] Example 3 : MIP protocol for exon trapping using 474 MIPs of variable length (20-30 nucleotides) with X and Y with equilibrated melting temperature (Tm).
[0084] In this example, the MIP probes utilized had variable X and Y region lengths, between 20-30 nucleotides. In this embodiment, Tm is calculated using a standard formula such that the X and Y melting temperatures are nearly equal.
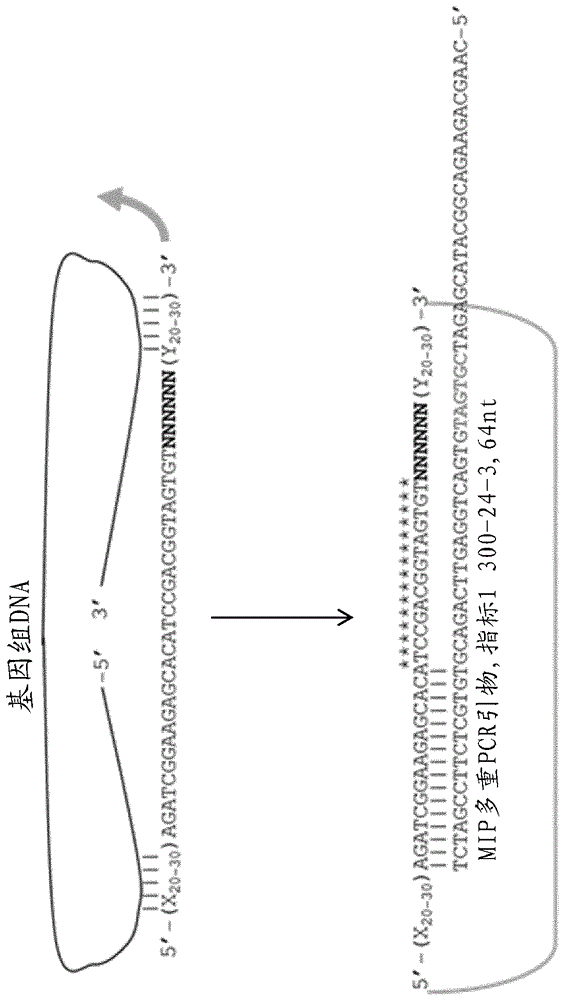
[0085] In the previous examples, MIP probes were prepared with a fixed-length 20-nucleotide target-specific region represented as follows:
[0086] 5'-(X20)AGATCGGAAGAGCACATCCGACGGTAGTGT(Y20), where X and Y represent two 20 nucleotide long target-specific regions. In this embodiment, a MIP probe has a variable region that can be represented as follows:
[0087] 5'-(X20-30) AGATCGGAAGAGCACATCCGACGGTAGTGT (Y20-30), wherein the X region and the Y region do not necessarily have the same length. exist Figure 5 Tm distributions of fixed-length 20-nucleotide probes and Tm-balanced 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com