An amphiphilic four-armed star polymer and its shell-based reversible cross-linked micelle system, preparation method and application
A four-armed star-shaped, polymer technology, applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of reducing the therapeutic effect and hindering the release of inner core drugs, etc., to reduce the Burst release phenomenon, realization of application value, effect of improving load and release efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
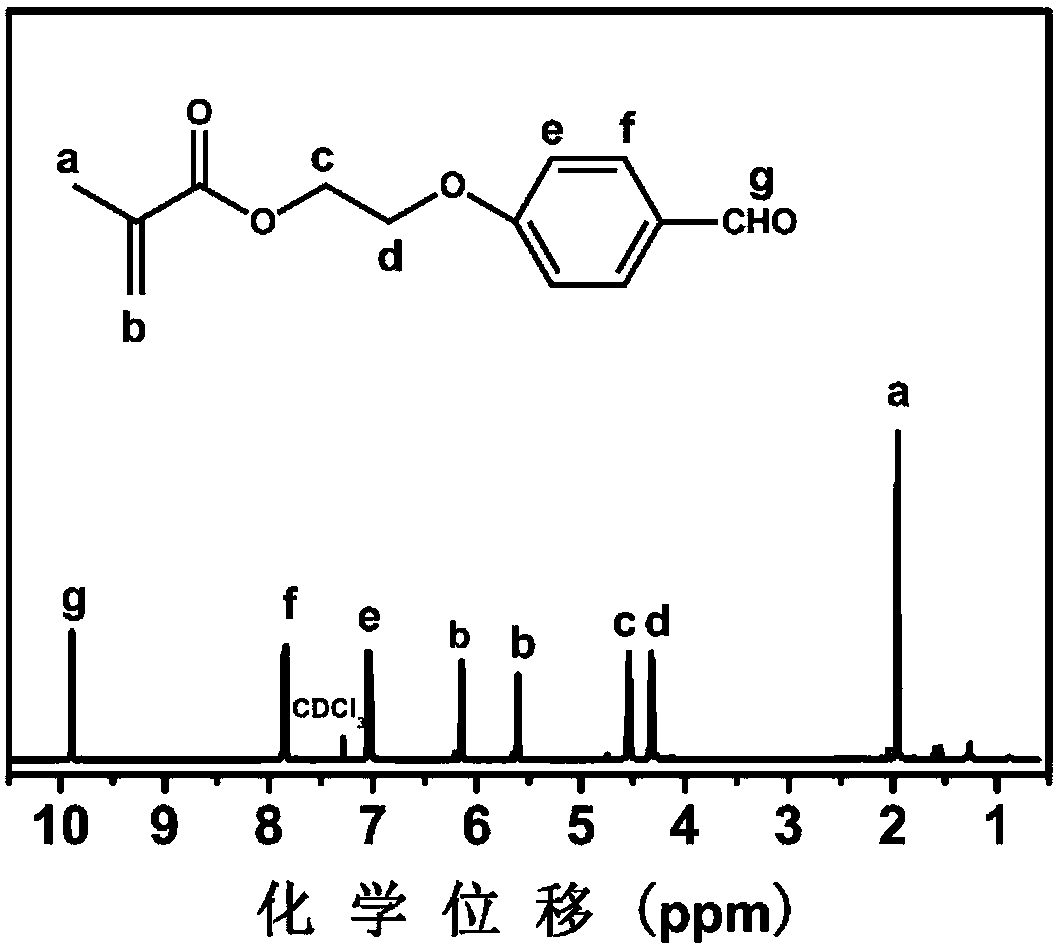
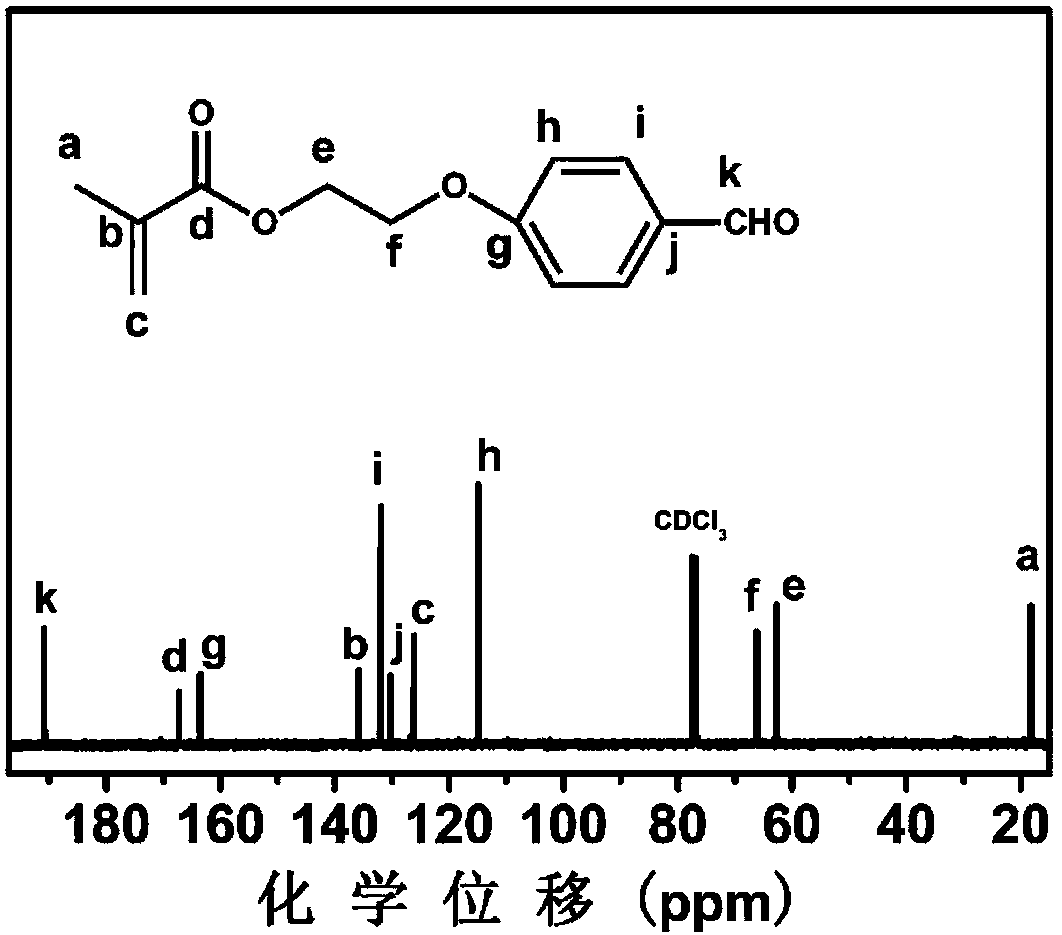
[0083] Example 1: Aldehyde functionalized monomer MAEBA
[0084] Take a 150mL clean three-necked flask, add a stirring bar, and under nitrogen protection, add p-(2-hydroxyethoxy)benzaldehyde (3.8g, 23mmol), triethylamine (2.33g, 23mmol) and 75mL di Chloromethane, ice bath, then slowly drop into 25mL dichloromethane containing methacryloyl chloride (7.2g, 69mmol), return to room temperature after stirring for 1h, continue to stir and react for 24h, pour into a separatory funnel, add H 2 O (100mL) extraction, then rinse the organic phase twice with NaCl (0.5M, 100mL) solution, then dry with anhydrous magnesium sulfate, filter, and remove the solvent by rotary evaporation, the obtained crude product is purified by silica gel column chromatography (eluent For n-hexane / ethyl acetate 4:1, R f value 0.4), and finally collect the target filtrate, and remove the solvent by rotary evaporation to obtain a white solid. The synthetic reaction formula is shown in formula (1). Analysis of...
Embodiment 2
[0086] Example 2: Aldehyde functionalized monomer MAEBA
[0087] Take a 150mL clean three-necked flask, add a stirring bar, and under nitrogen protection, add p-(2-hydroxyethoxy)benzaldehyde (3.8g, 23mmol), triethylamine (6.99g, 69mmol) and 75mL di Chloromethane, ice bath, then slowly drop into 25mL dichloromethane containing methacryloyl chloride (2.4g, 23mmol), return to room temperature after stirring for 5h, continue to stir and react for 12h, pour into a separatory funnel, add H 2 O (100mL) extraction, then rinse the organic phase twice with NaCl (0.5M, 100mL) solution, then dry with anhydrous magnesium sulfate, filter, and remove the solvent by rotary evaporation, the obtained crude product is purified by silica gel column chromatography (eluent For n-hexane / ethyl acetate 4:1, R f value 0.4), and finally collect the target filtrate, and remove the solvent by rotary evaporation to obtain a white solid.
Embodiment 3
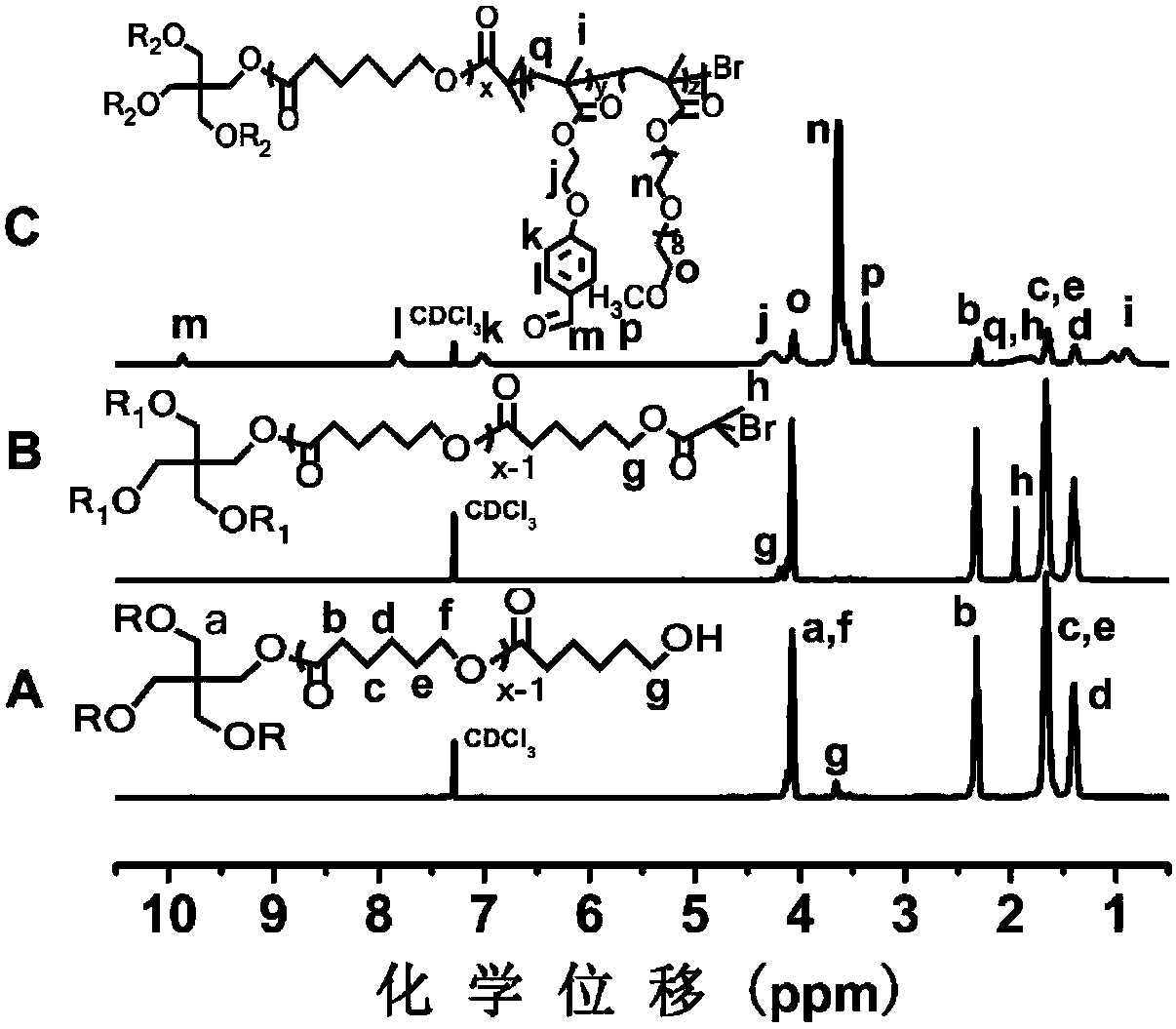
[0088] Example 3: Preparation of amphiphilic four-arm star polymer (4-AS-PCL-P(PEGMA-co-MAEBA)) (x:y:z=12:9:9)
[0089] (1) Preparation of polycaprolactone-containing polymer 4-AS-PCL
[0090] Take a 50mL dry reaction bottle, put it into a stirring bar, then bake the reaction bottle with an alcohol lamp for 5min, add the initiator pentaerythritol (136mg, 1mmol) into the reaction bottle, and seal it with a rubber stopper. Vacuumize - pass argon three times, add monomer ε-CL (5.47g, 48mmol) under the protection of argon. Heating in the bath for 20min, adding Sn(Oct) 2 (11 mg), after 16 h of reaction, the reaction was terminated with liquid nitrogen. Add 50 mL of THF to dissolve the polymer, precipitate with 500 mL of cold methanol / water (1:1), and dry under vacuum at 45 °C and 35 mb. The synthetic reaction formula is shown in formula (2). Using NMR, infrared and GPC to analyze the molecular structure and composition, the results are shown in image 3 , Figure 4 with Fig...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com