Ginkgo leaf total flavone controlled-release tablet and preparation method thereof
A technology of total flavonoids and ginkgo leaves, which is applied in the field of controlled-release tablets and preparation of total flavonoids from ginkgo leaves, which can solve the problems of cumbersome coating of controlled-release tablets and adverse health effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] prescription:
[0035] Ginkgo biloba total flavonoids 200mg
[0036] PEO-301 145mg
[0037] Methylcellulose 50mg
[0038] Lactose 90mg
[0040] Preparation method:
[0041] Pass the total flavonoids of Ginkgo biloba leaves, polyoxyethylene, methylcellulose, and lactose through 80-mesh sieves (grind first if necessary), weigh each excipient according to the prescription amount, mix them with total flavonoids of Ginkgo biloba leaves, and press into tablets. have to.
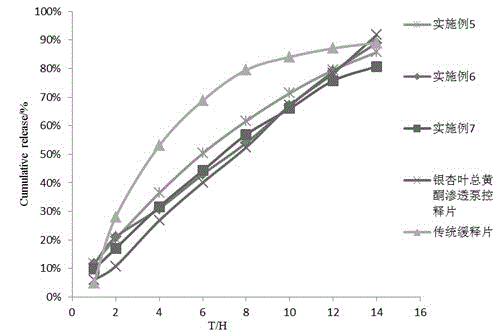
[0042] The correlation coefficient r measured by in vitro release curve was 0.9904.
Embodiment 2
[0044] prescription:
[0045] Ginkgo biloba total flavonoids 200mg
[0046] PEO-N10 70mg
[0047] Kabham 130mg
[0048] Microcrystalline Cellulose 80mg
[0050] Preparation method:
[0051] Pass the total flavonoids of Ginkgo biloba leaves, polyoxyethylene, carbomer, and microcrystalline cellulose through an 80-mesh sieve (grind first if necessary), weigh each auxiliary material and total flavonoids of Ginkgo biloba leaves according to the prescription amount, and use the method of equal addition Mix evenly, then add absolute ethanol to granulate, dry at 45°C for 12 hours, add magnesium stearate, mix evenly, and press into tablets to obtain.
[0052] The correlation coefficient r measured by in vitro release curve was 0.980.
Embodiment 3
[0054] prescription:
[0055] Ginkgo biloba total flavonoids 200mg
[0056] PEO-N60K 90mg
[0057] Hydroxypropyl Cellulose 60mg
[0058] Microcrystalline Cellulose 80mg
[0060] Preparation method:
[0061] Pass the total flavonoids of Ginkgo biloba leaves, polyoxyethylene, hydroxypropyl cellulose, and microcrystalline cellulose through 80-mesh sieves (if necessary, grind them first), weigh each auxiliary material and total flavonoids of Ginkgo biloba leaves according to the prescription amount, and weigh Mix by incremental method, then add 90% ethanol to granulate, dry at 45°C for 12 hours, add magnesium stearate, mix evenly, and press into tablets.
[0062] The correlation coefficient r measured by in vitro release curve was 0.985.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com