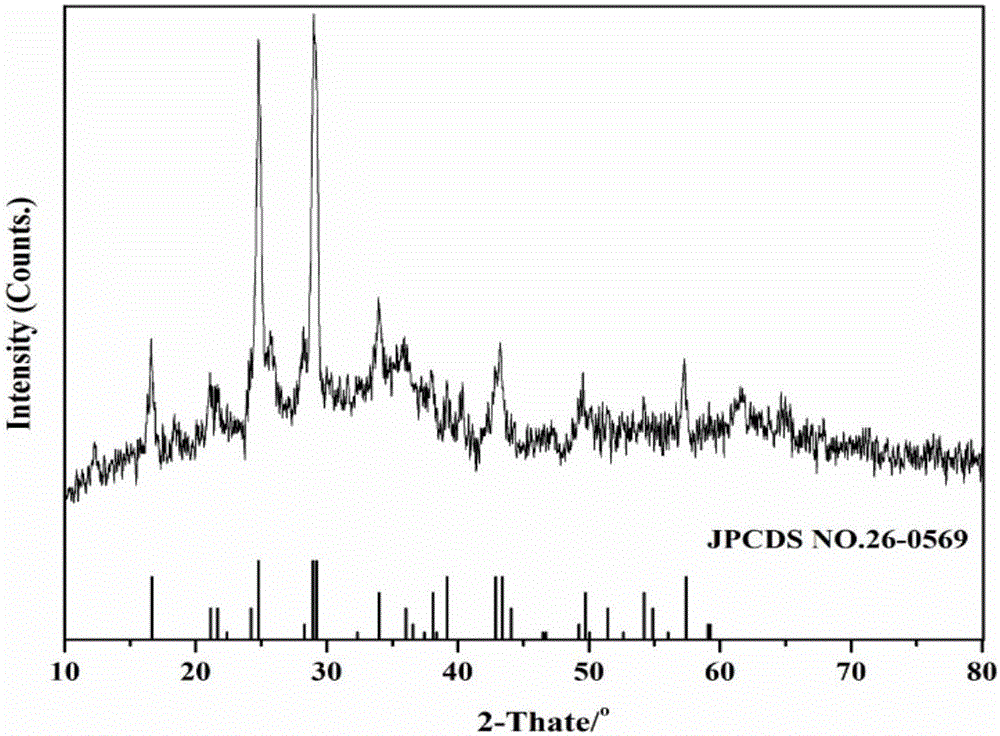
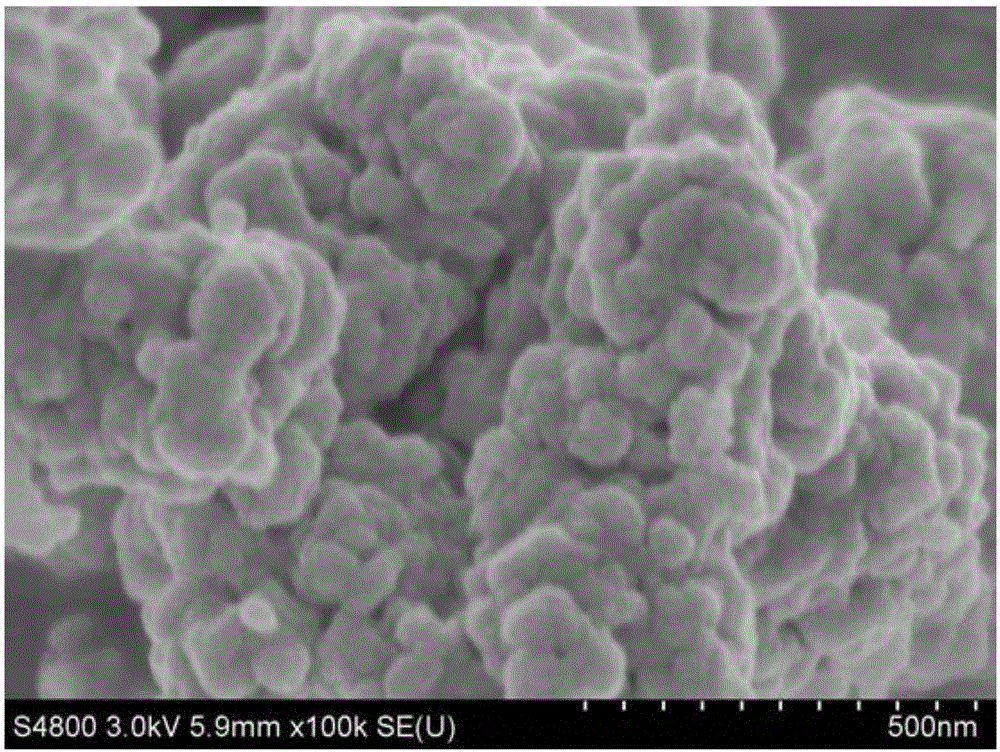
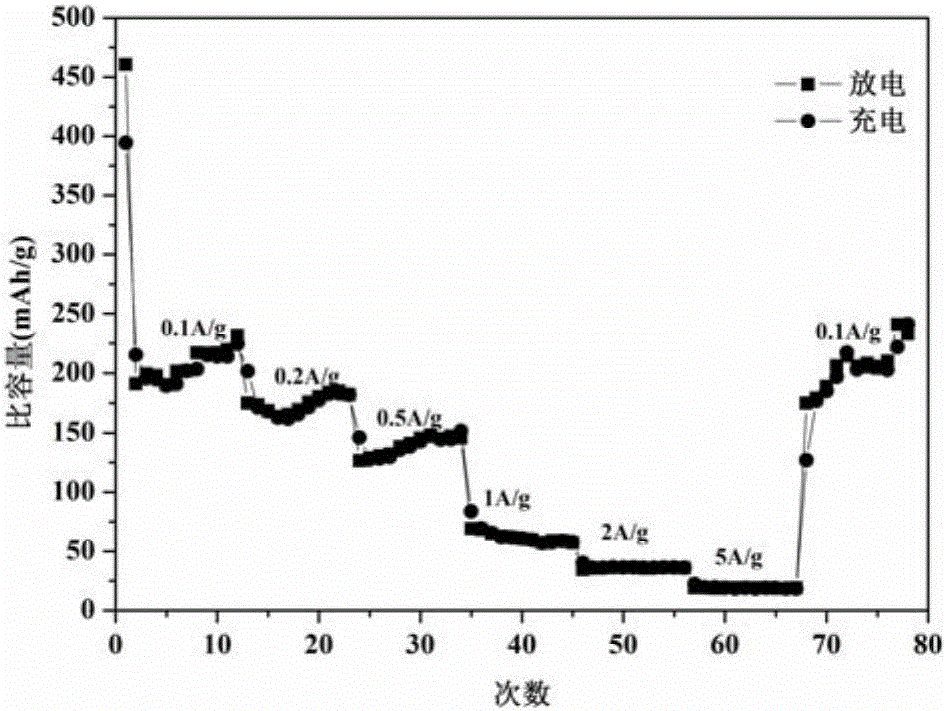
Preparation method of copper vanadate for lithium-ion battery cathode material
A technology for lithium-ion batteries and negative electrode materials, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as difficulty in large-scale industrial production, uncontrollable product morphology, long-term high-temperature treatment, etc., to achieve rich surface pores, Excellent electrochemical performance and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1) Weigh 0.7154g analytically pure cuprous oxide (Cu 2 O) dispersed in 100mL ethanol aqueous solution (V 乙醇 :V 水 =1:3), magnetically stirred for 0.5h to obtain suspension A. Weigh 0.91g analytically pure vanadium pentoxide (V 2 o 5 ) powder into 60mL hydrogen peroxide (H 2 o 2 ) aqueous solution, stirred for 0.1 h to obtain solution B; wherein, the volume fraction of hydrogen peroxide aqueous solution was 5%.
[0027] 2) After the solution B is stirred, immediately drop it into the suspension A drop by drop, and then continue to stir, the color of the solution A gradually changes from dark red to olive green. After stirring, filter, wash and dry under vacuum at 60°C for 12h.
[0028] 3) Spread the dried powder evenly in a porcelain boat, and under the protection of nitrogen, raise the temperature from room temperature to 300°C at a rate of 5°C / min and keep it warm for 0.5h; then cool with the furnace, and the obtained powder is lithium Ion battery anode copper v...
Embodiment 2
[0034] 1) Disperse analytically pure cuprous oxide in aqueous ethanol, and magnetically stir for 2 hours to disperse cuprous oxide in aqueous ethanol to obtain suspension A; wherein, the ratio of cuprous oxide to aqueous ethanol is 0.5:100mL; aqueous ethanol is Prepared by uniformly mixing deionized water and ethanol at a volume ratio of 2:1; adding analytically pure vanadium pentoxide powder into a 5% hydrogen peroxide aqueous solution, and stirring for 1.5 hours to obtain solution B; Wherein, the ratio of vanadium pentoxide powder to hydrogen peroxide aqueous solution is 1g:60mL.
[0035] 2) Immediately drop solution B into suspension A drop by drop, and then continue to stir until the color of the solution changes from dark red to olive green, stop stirring, after stirring, filter, wash and dry in vacuum at 60°C for 12 hours to obtain powder Body; Wherein, the mol ratio of the cuprous oxide in the suspension A and the vanadium pentoxide in the solution B is 0.5:1;
[0036]...
Embodiment 3
[0038] 1) Disperse analytically pure cuprous oxide in aqueous ethanol, and magnetically stir for 4 hours to disperse cuprous oxide in aqueous ethanol to obtain suspension A; wherein, the ratio of cuprous oxide to aqueous ethanol is 1g:100mL; aqueous ethanol is Prepared by uniformly mixing deionized water and ethanol at a volume ratio of 3:1; adding analytically pure vanadium pentoxide powder into a 5% hydrogen peroxide aqueous solution and stirring for 0.8h to obtain solution B; Wherein, the ratio of vanadium pentoxide powder to hydrogen peroxide aqueous solution is 0.5g:60mL.
[0039] 2) Immediately drop solution B into suspension A drop by drop, then continue to stir until the color of the solution changes from dark red to olive green, stop stirring, after stirring, filter and wash and dry in vacuum at 60°C for 12 hours to obtain powder Body; Wherein, the mol ratio of the cuprous oxide in the suspension A and the vanadium pentoxide in the solution B is 1:1;
[0040] 3) Unde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com