Structure, preparation method and application of isothiocyanate-type precursor compound
A technology of isothiocyanate and compounds, applied in organic chemistry, drug combination, antineoplastic drugs, etc., can solve the problem of non-existence of isothiocyanate, and achieve stable structure, low drug resistance, and small toxic and side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] The following examples can enable those skilled in the art to understand the present invention more fully, but do not limit the present invention in any way.
[0048] The general formulas of the active form (I) of the drug and its prodrug (II) are shown below:
[0049]
[0050] where R 1 for: C 4 -C 6 Alkyl, C 1 -C 4 Alkoxy, C 1 -C 6 Alkenyl, C 2 -C 10 Alkynyl, C 1 -C 4 Alkanoyl, C 1 -C 4 alkanoyloxy,
[0051] R 2 for:
[0052] R 3 For: Cl, Br, alkyl, C 1 -C 6 Alkenyl, C 2 -C 10 Alkynyl, C 1 -C 4 Alkanoyl, C 1 -C 4 Alkanoyloxy; R 4 For: H, OH, CF 3 or methoxy;
[0053] X is: O, S or NH; n=0,1,2.
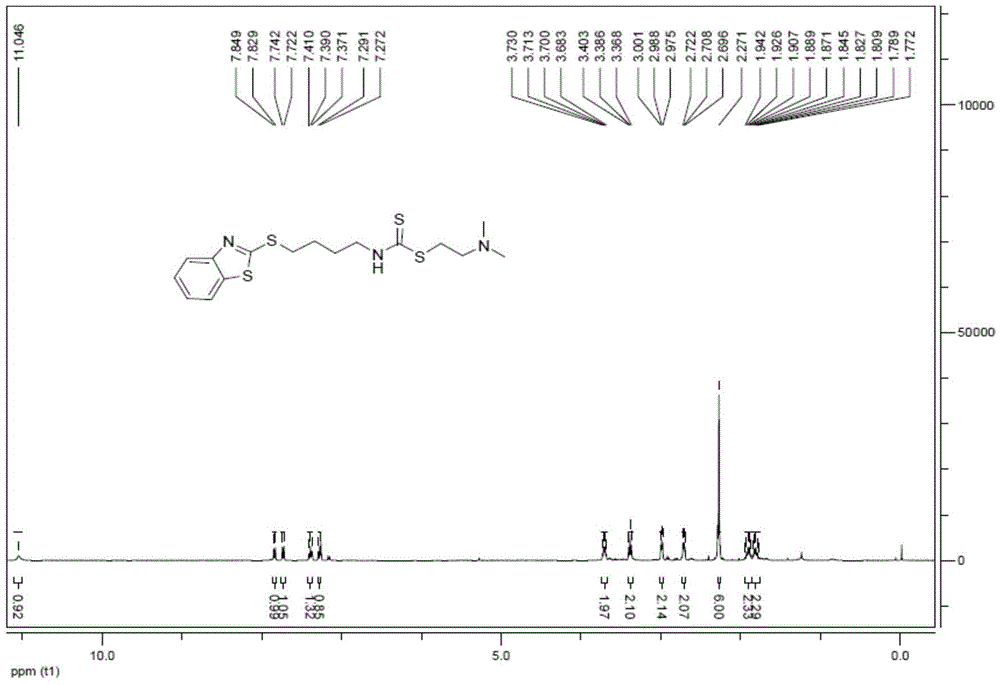
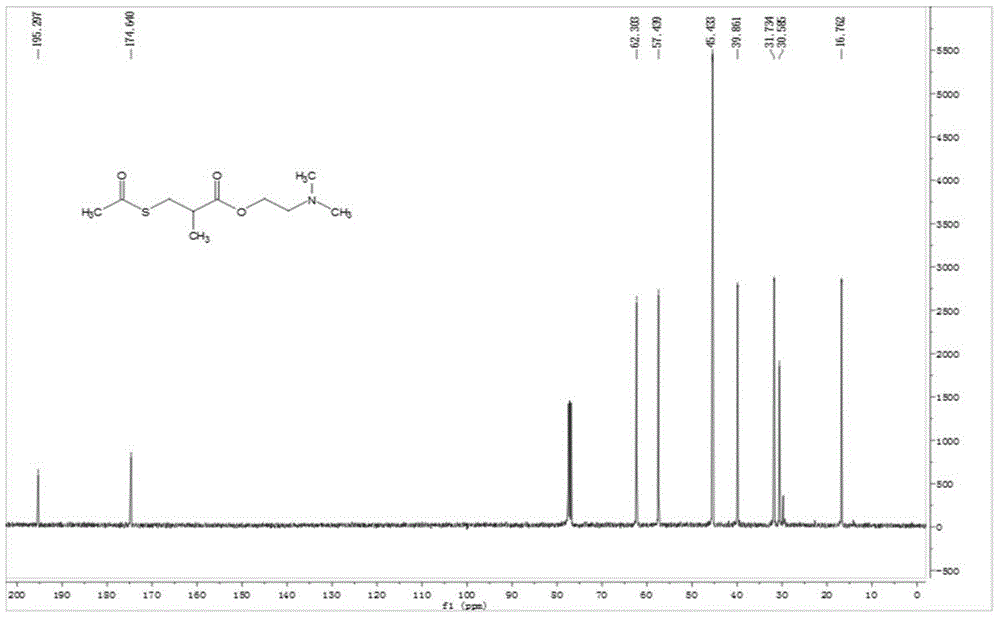
[0054] The synthetic steps and maps of various compounds are described below. The synthesis process of dimethylamino-(phenyl-1H-tetrazolium) isothiocyanate conjugate (represented by compound 1) is as follows:
[0055]
[0056] Each step is described step by step below.
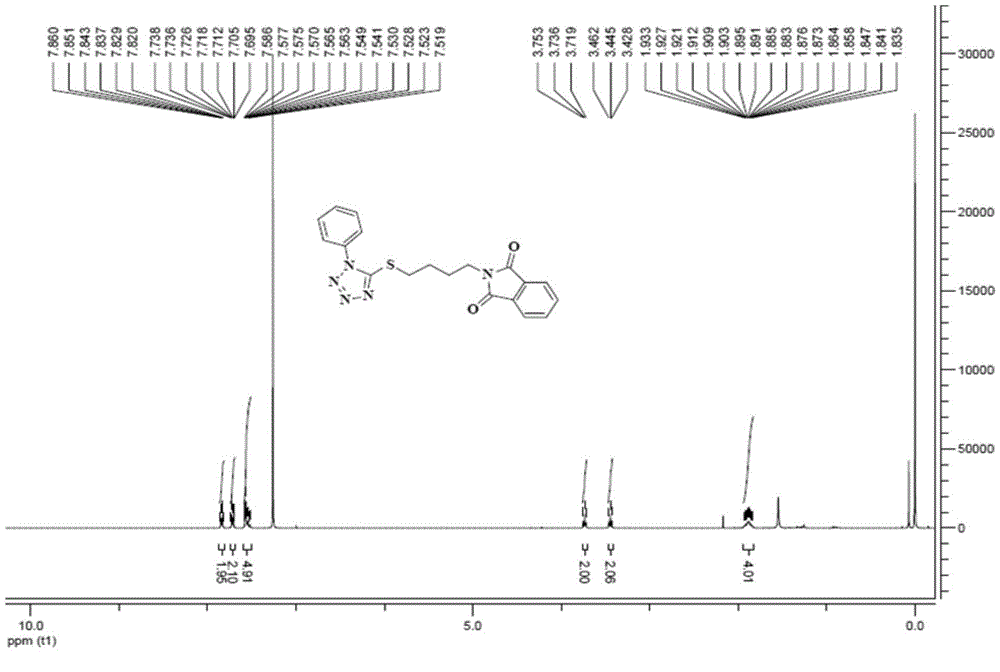
[0057] Synthesis of 2-[4-((1-phenyl-1H-tetrazol-5-yl)thio)butyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com