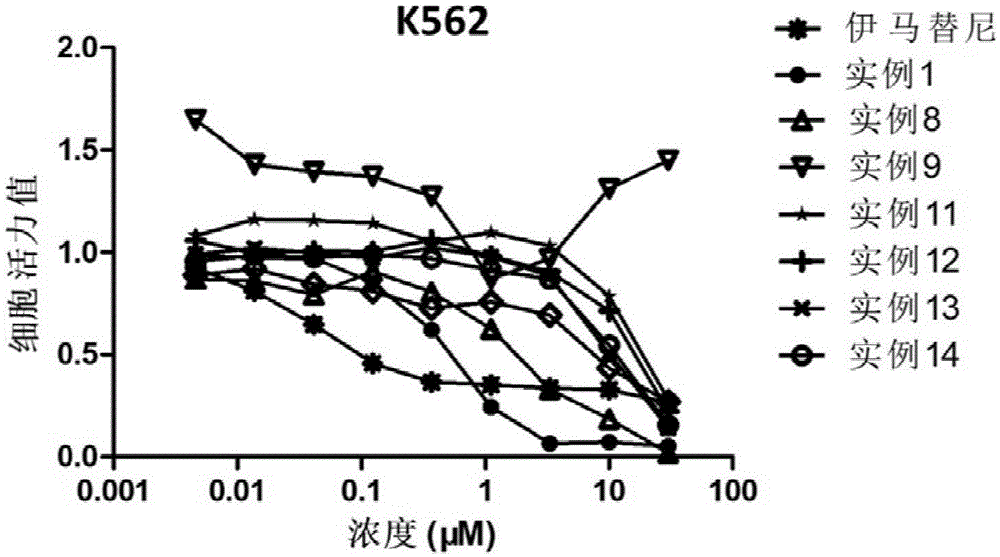
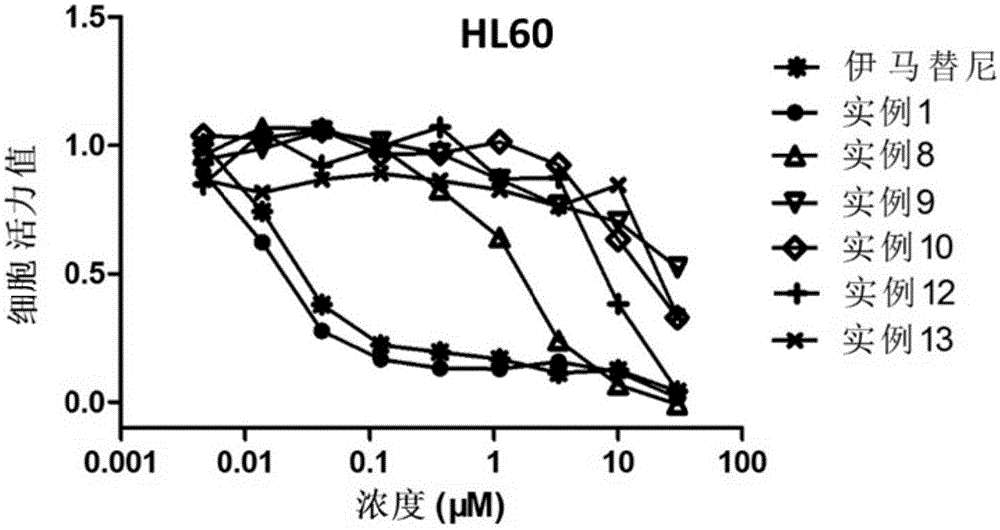
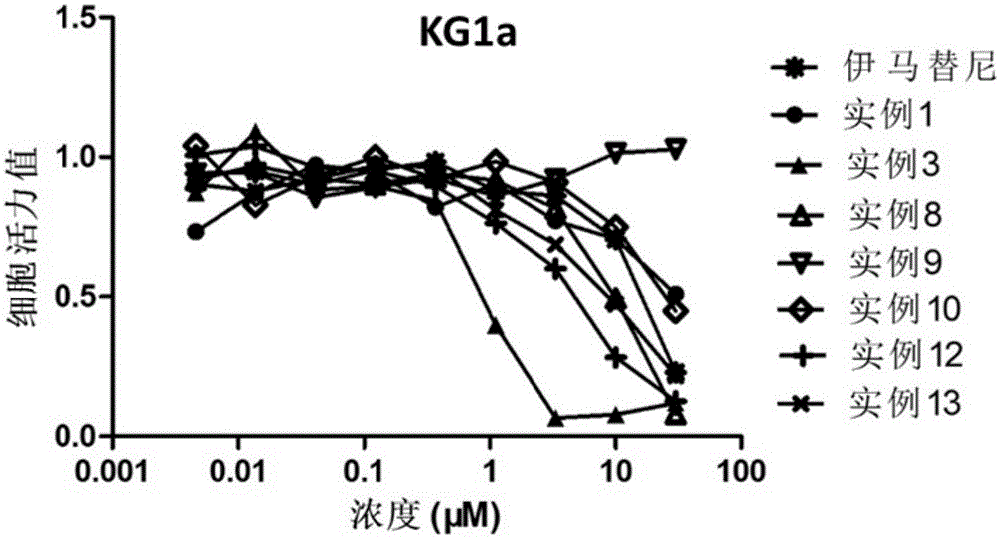
Heterocycle compound containing triazole, as well as preparation method and application of heterocycle compound
A technology of heterocycles and compounds, which is applied in the field of triazole-containing heterocycles and their preparation and application, and can solve the problems of poor curative effect of leukemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Chemical Synthesis and Structure Identification of Triazole-containing Heterocyclic Compounds
[0059] 1. The triazole-containing heterocyclic compound provided by the invention has the following general structural formula:
[0060]
[0061] In the above general structural formula, the substituent R includes the following structures:
[0062]
[0063] 2. The chemical synthesis experimental steps of the triazole-containing heterocyclic compounds provided by the present invention
[0064] 2.1 The general synthetic route is:
[0065]
[0066] 2.2 Experimental part
[0067] 2.2.1 Synthesis of compound 4-methyl-2-[(4-pyridin-3-yl-pyrimidin-2-yl)-amino]benzene azide (2)
[0068]
[0069] Take a 100ml three-necked round-bottom flask, and add N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-aminopyrimidine (reaction substrate 1, 500mg) in sequence at 0°C , sodium nitrite (250mg), water (15ml) (note that it is best to use mechanical stirring at this time, bec...
example 1-14
[0104] Synthesis of Example 1-14 Compounds 15a-15n
[0105]
example 1
[0106] Synthesis and structure identification of example 1 compound 15a
[0107]
[0108] Get 25ml three-neck round bottom flask, at room temperature, add ethanol-water mixed system (V 乙醇 :V 水 =2:1, 15ml), the obtained compound 5a (0.18g, 0.70mmol), the obtained compound 2 (0.25g, 0.83mmol), then add nano Cu 2 O (0.19g, 1.00mmol), reacted for 12 hours, and monitored the reaction progress by TLC during the reaction. After the reaction was completed, the reaction solution was filtered, concentrated, and purified by silica gel column chromatography to obtain 0.23 g of the product, namely compound 15a. MS of the compound: [M+H] + 554.54; 1 H NMR (400MHz, CDCl 3 )δppm 9.46(m, 1H), 9.12(s, 1H), 8.76-8.74(m, 1H), 8.60(d, J=4.4Hz, 1H), 8.44(d, J=7.2Hz, 2H), 8.30 (s, 1H), 8.16(s, 1H), 7.61(s, 1H), 7.51(d, J=7.2Hz, 2H), 7.41(d, J=8.0Hz, 1H), 7.30(s, 1H) , 7.25(s, 1H), 2.49(s, 3H), 2.36(s, 3H). 13 C NMR (100MHz, CDCl 3 )δ 162.7, 160.0, 159.3, 151.9, 148.6, 145.9, 138.8, 135.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com