Application and preparation method of ceramic membrane with chiral Salen catalysis function
A technology of functional ceramics and ceramic membranes, applied in catalytic reactions, chemical instruments and methods, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of low immobilization rate, insufficient reaction, conversion rate and ee value low level problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
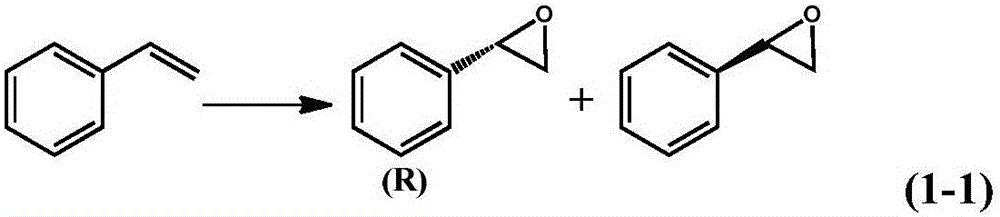
Examples
Embodiment 1
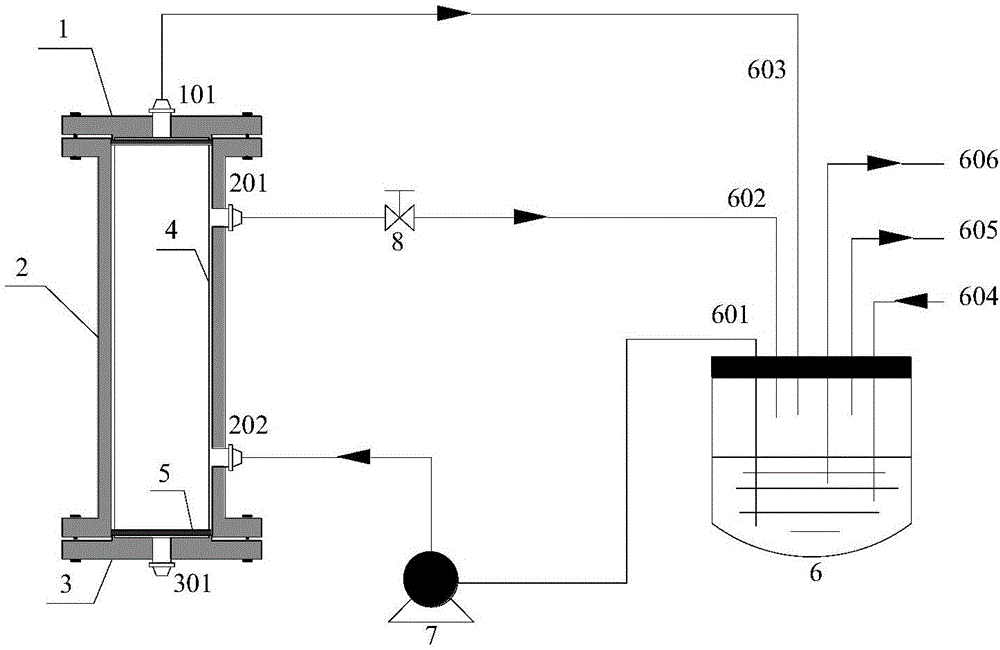
[0036] A kind of preparation method with chiral Salen catalytic function ceramic membrane, such as figure 2 As shown, the specific steps are as follows:
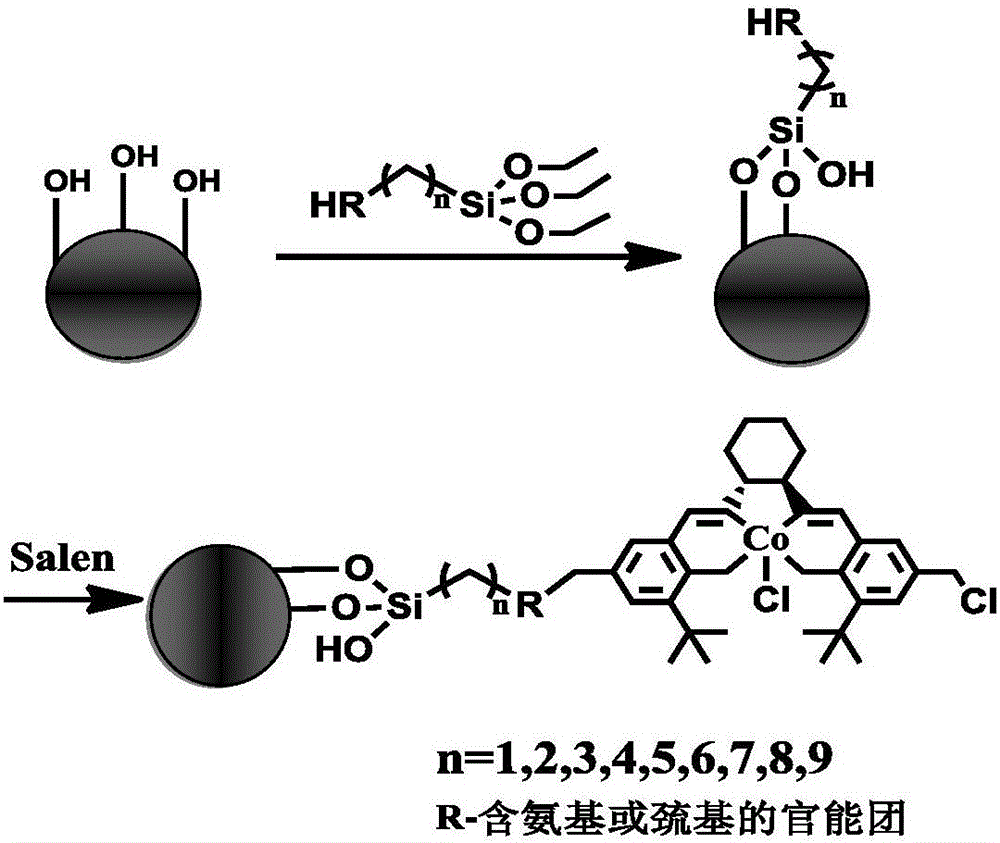
[0037] Step 1, mono-CH 2 Synthesis of Cl-Salen-Mn(Ⅲ) catalyst:
[0038] 3-tert-butyl salicylaldehyde (product 1) is synthesized from 2-tert-butylphenol, and 3-tert-butyl salicylaldehyde is chloromethylated to synthesize 3-tert-butyl-5-chloromethyl salicylaldehyde ( Product 2); Synthesis of 3,5-di-tert-butyl salicylaldehyde (product 3) from 2,4-di-tert-butylphenol via Duff reaction, product 3 and (1R,2R)-(-)-cyclohexanedi Amine reaction to obtain (R,R)-N-3,5-di-tert-butyl salicylaldehyde-1,2-cyclohexanediamine (product 4); reaction of product 4 with product 2 to obtain (R,R)- N-(3,5-di-tert-butylsalicylaldehyde)-N-(3'-tert-butyl-5'-chloromethylsalicylaldehyde)-1,2-cyclohexanediamine (product 5), Product 5 and Mn(OAc) 2 .4H 2 O reflux reaction makes (R, R)-N-(3,5-di-tert-butyl salicylaldehyde)-N-(3'-tert-butyl-5'-chloro...
Embodiment 2
[0051] A method for preparing a ceramic membrane with chiral Salen catalytic function, the specific steps are as follows:
[0052] Step 1, mono-CH 2 Synthesis of Cl-Salen-Co(Ⅲ) catalyst:
[0053] 3-tert-butyl salicylaldehyde (product 1) is synthesized from 2-tert-butylphenol, and 3-tert-butyl salicylaldehyde is chloromethylated to synthesize 3-tert-butyl-5-chloromethyl salicylaldehyde ( Product 2); Synthesis of 3,5-di-tert-butyl salicylaldehyde (product 3) from 2,4-di-tert-butylphenol via Duff reaction, product 3 and (1R,2R)-(-)-cyclohexanedi Amine reaction to obtain (R,R)-N-3,5-di-tert-butyl salicylaldehyde-1,2-cyclohexanediamine (product 4); reaction of product 4 with product 2 to obtain (R,R)- N-(3,5-di-tert-butylsalicylaldehyde)-N-(3'-tert-butyl-5'-chloromethylsalicylaldehyde)-1,2-cyclohexanediamine (product 5), Product 5 and Co(OAc) 2 .4H 2 O reflux reaction makes (R, R)-N-(3,5-di-tert-butyl salicylaldehyde)-N-(3'-tert-butyl-5'-chloromethyl salicylaldehyde)-1, 2-cyc...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap