Preparation method of carbon fiber loaded titanium dioxide nanometer sheet
A titanium dioxide and nanosheet technology, which is applied in the field of preparation of carbon fiber-supported titanium dioxide nanosheets, can solve the problems of high cost, hidden dangers of production safety, strong volatility, corrosion and the like, achieves simple preparation method, solves recycling difficulties, and improves photocatalysis. active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 3mL of butyl titanate, 15mL of concentrated hydrochloric acid and 15mL of deionized water into a 50mL reaction kettle lined with polytetrafluoroethylene, stir well, and then add 50mg of carbon fiber and 0.88g of sodium fluoroborate. The molar ratio of carbon fiber, titanium source, sodium fluoroborate, hydrochloric acid and deionized water is 1:2:2:22:200. The reaction kettle was placed at 180° C. for hydrothermal reaction for 12 hours. After the reaction is finished, the product can be prepared by washing and drying the carbon fiber-loaded titanium dioxide nanosheets.
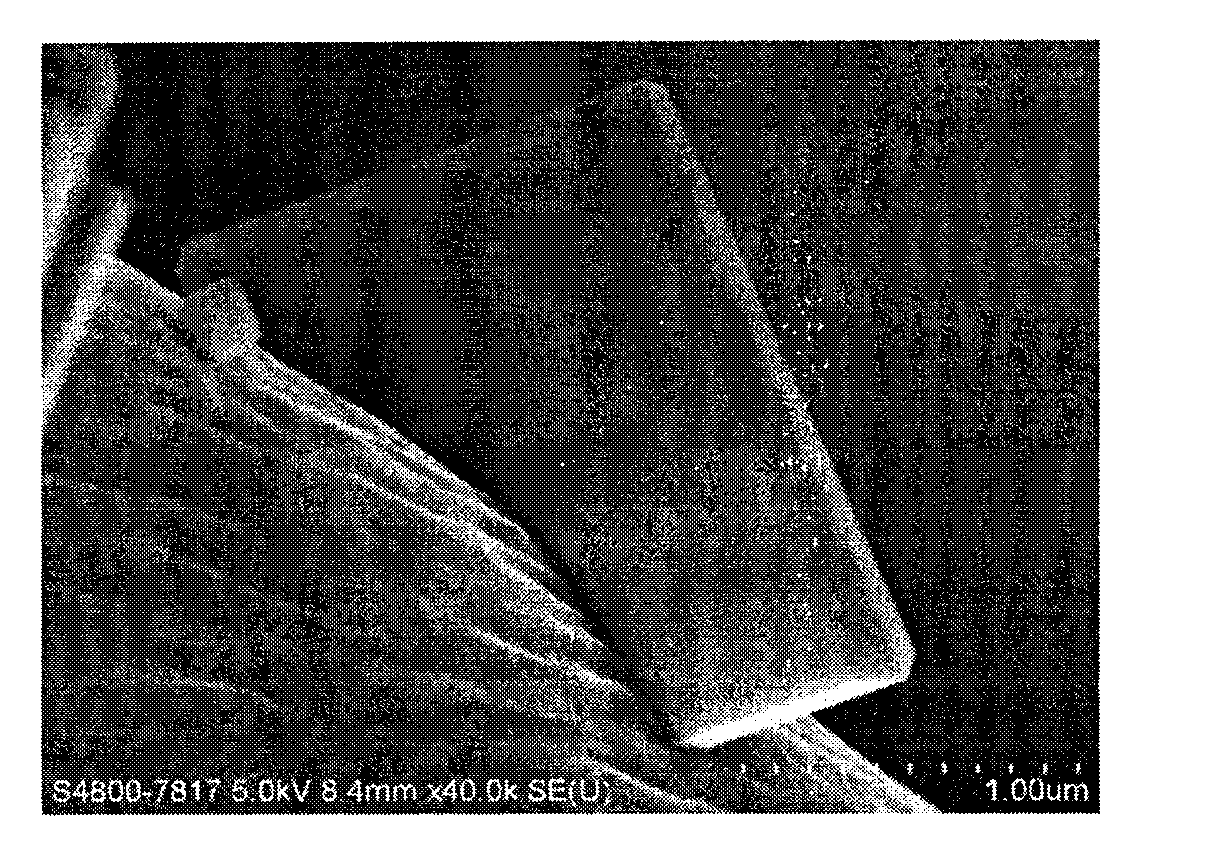
[0027] figure 1 It is a field emission scanning electron microscope image of the carbon fiber-supported titanium dioxide nano-flakes prepared in Example 1 of the present invention. It can be seen from the figure that the titanium dioxide nano-flakes are uniformly loaded on the carbon fibers.
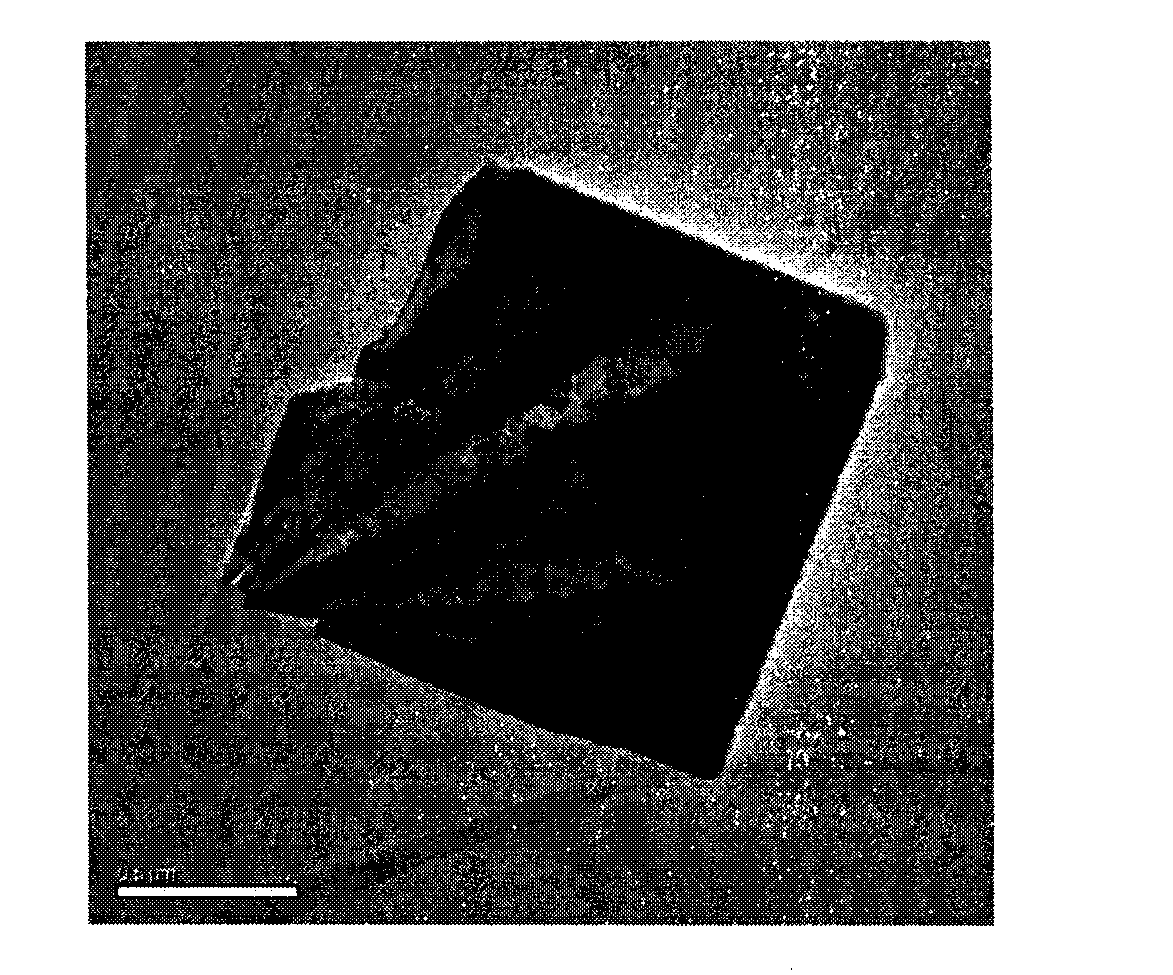
[0028] figure 2 It is the field emission scanning electron microscope image of the carbon fiber-loaded ...
Embodiment 2
[0032] Add 3mL of butyl titanate, 15mL of concentrated hydrochloric acid and 15mL of deionized water into a 50mL reactor lined with polytetrafluoroethylene, stir well, and then add 50mg of carbon fiber and 1.76g of sodium fluoroborate. The molar ratio of carbon fiber, titanium source, sodium fluoroborate, hydrochloric acid and deionized water is 1:2:4:22:200. The reaction kettle was placed at 180° C. for hydrothermal reaction for 12 hours. After the reaction is finished, the product can be prepared by washing and drying the carbon fiber-loaded titanium dioxide nanosheets.
[0033] Figure 5 It is a field emission scanning electron microscope of the carbon fiber-loaded titanium dioxide nano-flakes prepared in Example 2 of the present invention. It can be seen from the figure that the titanium dioxide nano-flakes are evenly loaded on the carbon fibers, and the side length is about 3.2 μm, and the thickness is about 303 nm. The calculated [001] surface exposure rate is about 61...
Embodiment 3
[0035] Add 3mL of butyl titanate, 15mL of concentrated hydrochloric acid and 15mL of deionized water into a 50mL reaction kettle lined with polytetrafluoroethylene, stir well, and then add 50mg of carbon fiber and 3.52g of sodium fluoroborate. The molar ratio of carbon fiber, titanium source, sodium fluoroborate, hydrochloric acid and deionized water is 1:2:8:22:200. The reaction kettle was placed at 180° C. for hydrothermal reaction for 12 hours. After the reaction is finished, the product can be prepared by washing and drying the carbon fiber-loaded titanium dioxide nanosheets.
[0036] Figure 6 It is the field emission scanning electron microscope image of the carbon fiber-loaded titanium dioxide nano-flakes prepared in Example 3 of the present invention. It can be seen from the figure that the titanium dioxide nano-flakes are uniformly loaded on the carbon fibers, with a side length of about 6.3 μm and a thickness of about 275 nm. The calculated [001] surface exposure r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com