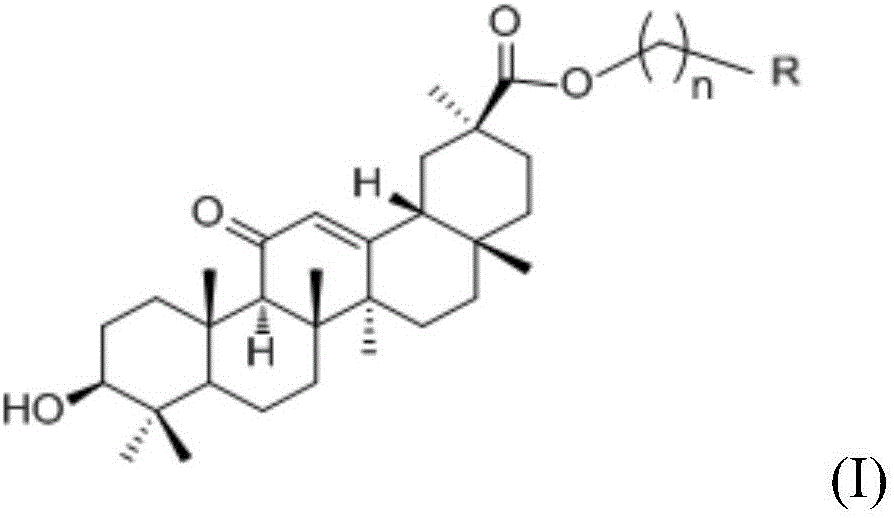
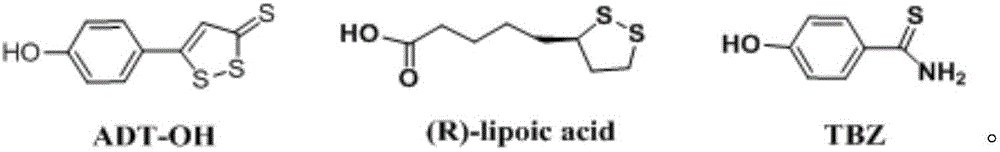
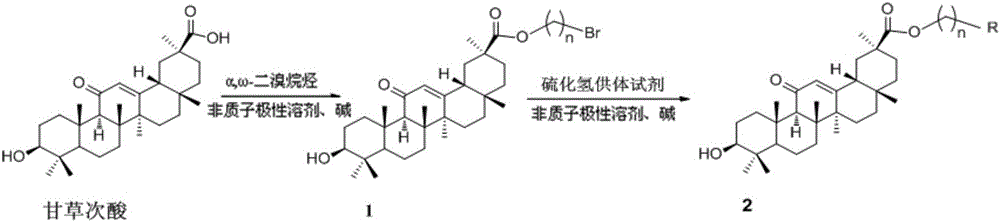
Glycyrrhetinic acid-hydrogen sulfide donor reagent derivative, and synthetic method and application thereof
A hydrogen sulfide donor, glycyrrhetic acid technology, applied in the field of medicine, can solve problems such as no synthesis method and application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the synthesis of compound 1a
[0042] Dissolve glycyrrhetinic acid (500mg, 1.06mmol) in anhydrous DMF (5mL), add 1,6-dibromoethane (2.43mL, 5.3mmol), K 2 CO 3 (146.28mg, 1.06mmol), reacted at 30°C for 24h. The solvent was evaporated under reduced pressure, and the residue was dispersed in ethyl acetate (50 mL), washed successively with HCl (1N), water, and saturated brine, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and separated by column chromatography (V PE :V EA =2:1), to obtain compound 1a (457mg, 75%, white solid).
[0043] Yield: 457mg, 75%, white solid; R f =0.461 (Petroluem ether:EtOAc=2:1).M.p 190-192°C. 1 H NMR (500MHz, CDCl 3)δ (ppm): 5.69 (s, 1H, 12-H), 4.41 (dd, J = 28.0, 5.9Hz, 2H, OCH 2 ), 3.53(t, J=5.7Hz, 2H, OCH 2 ),3.21(dd,J=11.0,5.2Hz,1H,3-H),2.83-2.71(m,1H,18-H),2.32(s,1H),2.21-0.63(m,20H),1.35 , 1.17, 1.12, 1.11, 0.99, 0.80 and 0.79 (7s, each 3H, 7×CH 3 ). 13 C NMR (1...
Embodiment 2
[0045] Embodiment 2: the synthesis of compound 1b
[0046] Dissolve glycyrrhetinic acid (500mg, 1.06mmol) in anhydrous DMF (5mL), add 1,8-dibromobutane (2.94mL, 5.3mmol), K 2 CO 3 (146.28mg, 1.06mmol), reacted at 30°C for 24h. The solvent was evaporated under reduced pressure, and the residue was dispersed in ethyl acetate (50 mL), washed successively with HCl (1N), water, and saturated brine, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and separated by column chromatography (V PE :V EA =2:1), to obtain compound 1b (532mg, 83%, white solid).
[0047] Yield: 532mg, 83%, white solid; R f =0.515 (Petroluem ether:EtOAc=2:1).M.p 82-84°C. 1 H NMR (500MHz, CDCl 3 )δ (ppm): 5.61 (s, 1H, 12-H), 4.12 (t, J = 6.3Hz, 2H, OCH 2 ), 3.43(t, J=6.5Hz, 2H, OCH 2 ), 3.21(dd, J=11.1, 5.1Hz, 1H, 3-H), 2.77(d, J=13.5Hz, 1H, 18-H), 2.32(s, 1H), 2.13-0.61(m, 24H ), 1.35, 1.14, 1.11, 1.10 and 0.98 (5s, each 3H, 5×CH 3 ),0.79(s,6H,2×CH ...
Embodiment 3
[0049] Embodiment 3: the synthesis of compound 1c
[0050] Dissolve glycyrrhetinic acid (1.0g, 2.12mmol) in anhydrous DMF (5mL), add 1,6-dibromohexane (1.62mL, 10.62mmol), K 2 CO 3 (293.0mg, 2.12mmol), reacted at 30°C for 24h. The solvent was evaporated under reduced pressure, the residue was dispersed in ethyl acetate (50ml), washed successively with HCl (1N), water, and saturated brine, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and separated by column chromatography (V PE :V EA =5:2), to obtain compound 1c (929mg, 69%, white solid).
[0051] Yield: 929mg, 69%, white solid; R f =0.452 (Petroluem ether:EtOAc=5:2).M.p 102-104°C. 1 H NMR (500MHz, CDCl 3 )δ (ppm): 5.61 (s, 1H, 12-H), 4.08 (m, 2H, OCH 2 ),3.39(m,2H,CH 2 -Br), 3.20(dd, J=11.1, 5.2Hz, 1H, 3-H), 2.76(d, J=13.5Hz, 1H, 18-H), 2.32(s, 1H, 10-H), 2.09 -0.69(m,35H),1.35,1.13,1.11,1.10and 0.98(5s,each 3H,5×CH 3 ),0.79(s,6H,2×CH 3 ). 13 C NMR (125MHz, CD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com